DNA damage alters DNA polymerase delta to a form that exhibits increased discrimination against modified template bases and mismatched primers
- PMID: 19074196
- PMCID: PMC2632934
- DOI: 10.1093/nar/gkn1000
DNA damage alters DNA polymerase delta to a form that exhibits increased discrimination against modified template bases and mismatched primers
Abstract
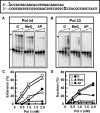
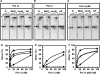
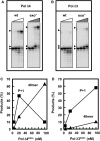
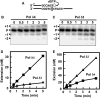
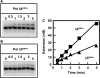
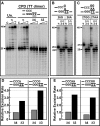
Human DNA polymerase delta (Pol delta4), a key enzyme in chromosomal replication, is a heterotetramer composed of the p125, p50, p68 and p12 subunits. Genotoxic agents such as UV and alkylating chemicals trigger a DNA damage response in which Pol delta4 is converted to a trimer (Pol delta3) by degradation of p12. We show that Pol delta3 has altered enzymatic properties: it is less able to perform translesion synthesis on templates containing base lesions (O(6)-MeG, 8-oxoG, an abasic site or a thymine-thymine dimer); a greater proofreading activity; an increased exonuclease/polymerase activity ratio; a decreased tendency for the insertion of wrong nucleotides, and for the extension of mismatched primers. Overall, our findings indicate that Pol delta3 exhibits an enhanced ability for the detection of errors in both primers and templates over its parent enzyme. These alterations in Pol delta3 show that p12 plays a major role in Pol delta4 catalytic functions, and provides significant insights into the rationale for the conversion of Pol delta4 to Pol delta3 in the cellular response to DNA damage.
Figures
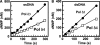


References
-
- Brutlag D, Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3′ to 5′ exonuclease activity in deoxyribonucleic acid polymerases. J. Biol. Chem. 1972;247:241–248. - PubMed
-
- Johnson A, O'Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu. Rev. Biochem. 2005;74:283–315. - PubMed
-
- Joyce CM, Steitz TA. Function and structure relationships in DNA polymerases. Annu. Rev. Biochem. 1994;63:777–822. - PubMed
-
- Kunkel TA, Bebenek K. DNA replication fidelity. Annu. Rev. Biochem. 2000;69:497–529. - PubMed
-
- Johnson KA. Conformational coupling in DNA polymerase fidelity. Annu. Rev. Biochem. 1993;62:685–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

