CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit
- PMID: 19074257
- PMCID: PMC2629307
- DOI: 10.1073/pnas.0810114105
CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit
Abstract
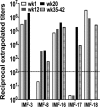
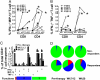
Blockade of inhibitory signals mediated by cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) has been shown to enhance T cell responses and induce durable clinical responses in patients with metastatic melanoma. The functional impact of anti-CTLA-4 therapy on human immune responses is still unclear. To explore this, we analyzed immune-related adverse events and immune responses in metastatic melanoma patients treated with ipilimumab, a fully human anti-CTLA-4 monoclonal antibody. Fifteen patients were selected on the basis of availability of suitable specimens for immunologic monitoring, and eight of these showed evidence of clinical benefit. Five of the eight patients with evidence of clinical benefit had NY-ESO-1 antibody, whereas none of seven clinical non-responders was seropositive for NY-ESO-1. All five NY-ESO-1 seropositive patients had clearly detectable CD4(+) and CD8(+) T cells against NY-ESO-1 following treatment with ipilimumab. One NY-ESO-1 seronegative clinical responder also had a NY-ESO-1 CD4(+) and CD8(+) T cell response, possibly related to prior vaccination with NY-ESO-1. Among five clinical non-responders analyzed, only one had a NY-ESO-1 CD4(+) T cell response and this patient did not have detectable anti-NY-ESO-1 antibody. Overall, NY-ESO-1-specific T cell responses increased in frequency and functionality during anti-CTLA-4 treatment, revealing a polyfunctional response pattern of IFN-gamma, MIP-1beta and TNF-alpha. We therefore suggest that CTLA-4 blockade enhanced NY-ESO-1 antigen-specific B cell and T cell immune responses in patients with durable objective clinical responses and stable disease. These data provide an immunologic rationale for the efficacy of anti-CTLA-4 therapy and call for immunotherapeutic designs that combine NY-ESO-1 vaccination with CTLA-4 blockade.
Conflict of interest statement
Conflict of interest statement: J.P.A. and J.D.W. are consultants to Bristol-Myers Squibb and Medarex. J.P.A. is an inventor of intellectual property related to anti-CTLA-4 that is held by the University of California and has been licensed to Medarex and Bristol-Myers Squibb.
Figures

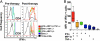
Comment in
-
No correlation between clinical response to CTLA-4 blockade and presence of NY-ESO-1 antibody in patients with metastatic melanoma.J Immunother. 2009 Oct;32(8):884-5. doi: 10.1097/CJI.0b013e3181affbf0. J Immunother. 2009. PMID: 19752745 Free PMC article. No abstract available.
References
-
- Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

