Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task
- PMID: 19074268
- PMCID: PMC2629291
- DOI: 10.1073/pnas.0810524105
Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task
Abstract
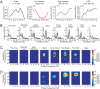
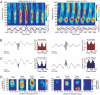
Oscillatory rhythms in different frequency ranges mark different behavioral states and are thought to provide distinct temporal windows that coherently bind cooperating neuronal assemblies. However, the rhythms in different bands can also interact with each other, suggesting the possibility of higher-order representations of brain states by such rhythmic activity. To explore this possibility, we analyzed local field potential oscillations recorded simultaneously from the striatum and the hippocampus. As rats performed a task requiring active navigation and decision making, the amplitudes of multiple high-frequency oscillations were dynamically modulated in task-dependent patterns by the phase of cooccurring theta-band oscillations both within and across these structures, particularly during decision-making behavioral epochs. Moreover, the modulation patterns uncovered distinctions among both high- and low-frequency subbands. Cross-frequency coupling of multiple neuronal rhythms could be a general mechanism used by the brain to perform network-level dynamical computations underlying voluntary behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Buzsaki G. Rhythms of the Brain. Oxford: Oxford Univ Press; 2006.
-
- Buzsaki G, et al. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116:201–211. - PubMed
-
- Hentschke H, Perkins MG, Pearce RA, Banks MI. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur J Neurosci. 2007;26:1642–1656. - PubMed
-
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

