Tissue-specific PKA inhibition using a chemical genetic approach and its application to studies on sperm capacitation
- PMID: 19074277
- PMCID: PMC2634883
- DOI: 10.1073/pnas.0810971105
Tissue-specific PKA inhibition using a chemical genetic approach and its application to studies on sperm capacitation
Abstract
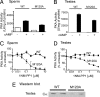
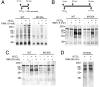
Studies on cAMP signaling and protein kinase A (PKA) function in vivo are limited by the lack of highly specific inhibitors that can be used in primary cell culture and whole animals. Previously we reported that a mutation in the ATP binding pocket of a catalytic subunit (Calpha) of PKA confers sensitivity to the pyrazolo[3,4-d]pyrimidine inhibitor, 1NM-PP1. We have now engineered the mouse Pkraca gene such that after Cre-mediated recombination in vivo, the CalphaM120A mutant protein is expressed and the wild-type Calpha is turned off. We demonstrate the utility of this approach by examining the requirement for PKA activity during capacitation of sperm from mice that express CalphaM120A mutant protein. For CalphaM120A sperm, 10 microM of 1NM-PP1 prevented PKA-dependent phosphorylation and the activation of motility that are both rapidly (<90 s) evoked by the HCO(3)(-) anion. A continuous (90 min) inhibition with 10 microM of 1NM-PP1 prevented the protein tyrosine phosphorylation of late-stage capacitation. Delayed application of 1NM-PP1 demonstrated that PKA activity was required for at least the initial 30 min of capacitation to produce subsequent protein tyrosine phosphorylation. Acute application of 1NM-PP1 rapidly slowed the accelerated beat of activated motility but did not affect the established waveform asymmetry of hyperactivated sperm. Our results demonstrate that PKA in CalphaM120A mutant sperm is rapidly and reversibly inhibited by 1NM-PP1 and that this blockade has selective and time-dependent effects on multiple aspects of capacitation. The conditional CalphaM120A-expressing mouse lines will be valuable tools for studying PKA function in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Understanding the molecular basis of sperm capacitation through kinase design.Proc Natl Acad Sci U S A. 2009 Jan 20;106(3):667-8. doi: 10.1073/pnas.0811895106. Epub 2009 Jan 14. Proc Natl Acad Sci U S A. 2009. PMID: 19144927 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

