Fluorescence detection of the movement of single KcsA subunits reveals cooperativity
- PMID: 19074286
- PMCID: PMC2629256
- DOI: 10.1073/pnas.0807056106
Fluorescence detection of the movement of single KcsA subunits reveals cooperativity
Abstract
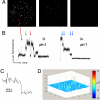
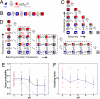
The prokaryotic KcsA channel is gated at the helical bundle crossing by intracellular protons and inactivates at the extracellular selectivity filter. The C-terminal transmembrane helix has to undergo a conformational change for potassium ions to access the central cavity. Whereas a partial opening of the tetrameric channel is suggested to be responsible for subconductance levels of ion channels, including KcsA, a cooperative opening of the 4 subunits is postulated as the final opening step. In this study, we used single-channel fluorescence spectroscopy of KcsA to directly observe the movement of each subunit and the temporal correlation between subunits. Purified KcsA channels labeled at the C terminus near the bundle crossing have been inserted into supported lipid bilayer, and the fluorescence traces analyzed by means of a cooperative or independent Markov model. The analysis revealed that the 4 subunits do not move fully independently but instead showed a certain degree of cooperativity. However, the 4 subunits do not simply open in 1 concerted step.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
-
- Shimizu H, et al. Global twisting motion of single molecular KcsA potassium channel upon gating. Cell. 2008;132:67–78. - PubMed
-
- Cordero-Morales JF, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

