Regulation and activity of a zinc uptake regulator, Zur, in Corynebacterium diphtheriae
- PMID: 19074382
- PMCID: PMC2648206
- DOI: 10.1128/JB.01392-08
Regulation and activity of a zinc uptake regulator, Zur, in Corynebacterium diphtheriae
Abstract
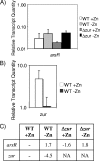
Regulation of metal ion homeostasis is essential to bacterial cell survival, and in most species it is controlled by metal-dependent transcriptional regulators. In this study, we describe a Corynebacterium diphtheriae ferric uptake regulator-family protein, Zur, that controls expression of genes involved in zinc uptake. By measuring promoter activities and mRNA levels, we demonstrate that Zur represses transcription of three genes (zrg, cmrA, and troA) in zinc-replete conditions. All three of these genes have similarity to genes involved in zinc uptake. Transcription of zrg and cmrA was also shown to be regulated in response to iron and manganese, respectively, by mechanisms that are independent of Zur. We demonstrate that the activity of the zur promoter is slightly decreased under low zinc conditions in a process that is dependent on Zur itself. This regulation of zur transcription is distinctive and has not yet been described for any other zur. An adjacent gene, predicted to encode a metal-dependent transcriptional regulator in the ArsR/SmtB family, is transcribed from a separate promoter whose activity is unaffected by Zur. A C. diphtheriae zur mutant was more sensitive to peroxide stress, which suggests that zur has a role in protecting the bacterium from oxidative damage. Our studies provide the first evidence of a zinc specific transcriptional regulator in C. diphtheriae and give new insights into the intricate regulatory network responsible for regulating metal ion concentrations in this toxigenic human pathogen.
Figures
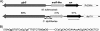
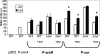



References
-
- Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27215-237. - PubMed
-
- Beard, S. J., M. N. Hughes, and R. K. Poole. 1995. Inhibition of the cytochrome bd-terminated NADH oxidase system in Escherichia coli K-12 by divalent metal cations. FEMS Microbiol. Lett. 131205-210. - PubMed
-
- Bibb, L. A., N. D. King, C. A. Kunkle, and M. P. Schmitt. 2005. Analysis of a heme-dependent signal transduction system in Corynebacterium diphtheriae: deletion of the chrAS genes results in heme sensitivity and diminished heme-dependent activation of the hmuO promoter. Infect. Immun. 737406-7412. - PMC - PubMed
-
- Bray, T. M., and W. J. Bettger. 1990. The physiological role of zinc as an antioxidant. Free Radic Biol. Med. 8281-291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

