IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression
- PMID: 19074392
- PMCID: PMC2631988
- DOI: 10.1128/JB.01086-08
IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression
Abstract
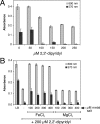
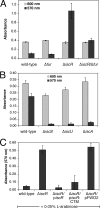
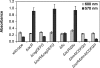
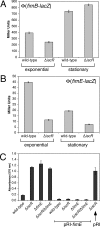
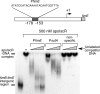
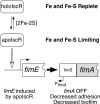
Biofilm formation is a complex developmental process regulated by multiple environmental signals. In addition to other nutrients, the transition metal iron can also regulate biofilm formation. Iron-dependent regulation of biofilm formation varies by bacterial species, and the exact regulatory pathways that control iron-dependent biofilm formation are often unknown or only partially characterized. To address this gap in our knowledge, we examined the role of iron availability in regulating biofilm formation in Escherichia coli. The results indicate that biofilm formation is repressed under low-iron conditions in E. coli. Furthermore, a key iron regulator, IscR, controls biofilm formation in response to changes in cellular Fe-S homeostasis. IscR regulates the FimE recombinase to control expression of type I fimbriae in E. coli. We propose that iron-dependent regulation of FimE via IscR leads to decreased surface attachment and biofilm dispersal under iron-limiting conditions.
Figures
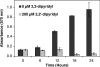

References
-
- Aberg, A., V. Shingler, and C. Balsalobre. 2006. (p)ppGpp regulates type 1 fimbriation of Escherichia coli by modulating the expression of the site-specific recombinase FimB. Mol. Microbiol. 601520-1533. - PubMed
-
- Aberg, A., V. Shingler, and C. Balsalobre. 2008. Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol. Microbiol. 671223-1241. - PubMed
-
- Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27215-237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

