Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1alpha: Targeting G6PT with mumbaistatin analogs in hypoxic mesenchymal stromal cells
- PMID: 19074414
- PMCID: PMC2728688
- DOI: 10.1634/stemcells.2008-0855
Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1alpha: Targeting G6PT with mumbaistatin analogs in hypoxic mesenchymal stromal cells
Abstract
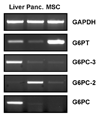
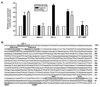
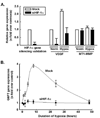
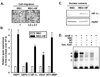
Mesenchymal stromal cell (MSC) markers are expressed on brain tumor-initiating cells involved in the development of hypoxic glioblastoma. Given that MSCs can survive hypoxia and that the glucose-6-phosphate transporter (G6PT) provides metabolic control that contributes to MSC mobilization and survival, we investigated the effects of low oxygen (1.2% O(2)) exposure on G6PT gene expression. We found that MSCs significantly expressed G6PT and the glucose-6-phosphatase catalytic subunit beta, whereas expression of the glucose-6-phosphatase catalytic subunit alpha and the islet-specific glucose-6-phosphatase catalytic subunit-related protein was low to undetectable. Analysis of the G6PT promoter sequence revealed potential binding sites for hypoxia inducible factor (HIF)-1alpha and for the aryl hydrocarbon receptor (AhR) and its dimerization partner, the AhR nuclear translocator (ARNT), AhR:ARNT. In agreement with this, hypoxia and the hypoxia mimetic cobalt chloride induced the expression of G6PT, vascular endothelial growth factor (VEGF), and HIF-1alpha. Gene silencing of HIF-1alpha prevented G6PT and VEGF induction in hypoxic MSCs whereas generation of cells stably expressing HIF-1alpha resulted in increased endogenous G6PT gene expression. A semisynthetic analog of the polyketide mumbaistatin, a potent G6PT inhibitor, specifically reduced MSC-HIF-1alpha cell survival. Collectively, our data suggest that G6PT may account for the metabolic flexibility that enables MSCs to survive under conditions characterized by hypoxia and could be specifically targeted within developing tumors.
Figures
Similar articles
-
LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning.Stem Cell Res Ther. 2018 Oct 25;9(1):280. doi: 10.1186/s13287-018-1031-x. Stem Cell Res Ther. 2018. PMID: 30359325 Free PMC article.
-
A concerted HIF-1α/MT1-MMP signalling axis regulates the expression of the 3BP2 adaptor protein in hypoxic mesenchymal stromal cells.PLoS One. 2011;6(6):e21511. doi: 10.1371/journal.pone.0021511. Epub 2011 Jun 27. PLoS One. 2011. PMID: 21738685 Free PMC article.
-
Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1α Hydroxylase Inhibitors.Stem Cells. 2018 Sep;36(9):1380-1392. doi: 10.1002/stem.2844. Epub 2018 Jun 8. Stem Cells. 2018. PMID: 29726060 Free PMC article.
-
NcoA2-Dependent Inhibition of HIF-1α Activation Is Regulated via AhR.Toxicol Sci. 2015 Dec;148(2):517-30. doi: 10.1093/toxsci/kfv199. Epub 2015 Sep 8. Toxicol Sci. 2015. PMID: 26350169
-
HIF-1α pathway: role, regulation and intervention for cancer therapy.Acta Pharm Sin B. 2015 Sep;5(5):378-89. doi: 10.1016/j.apsb.2015.05.007. Epub 2015 Jun 6. Acta Pharm Sin B. 2015. PMID: 26579469 Free PMC article. Review.
Cited by
-
Hypoxia-mimetic agents inhibit proliferation and alter the morphology of human umbilical cord-derived mesenchymal stem cells.BMC Cell Biol. 2011 Aug 9;12:32. doi: 10.1186/1471-2121-12-32. BMC Cell Biol. 2011. PMID: 21827650 Free PMC article.
-
Growth Factor-Reinforced ECM Fabricated from Chemically Hypoxic MSC Sheet with Improved In Vivo Wound Repair Activity.Biomed Res Int. 2017;2017:2578017. doi: 10.1155/2017/2578017. Epub 2017 Sep 5. Biomed Res Int. 2017. PMID: 29018809 Free PMC article.
-
Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cells.J Biol Chem. 2013 May 10;288(19):13378-86. doi: 10.1074/jbc.M113.456533. Epub 2013 Apr 2. J Biol Chem. 2013. PMID: 23548906 Free PMC article.
-
Interplay Between Glucose Metabolism and Chromatin Modifications in Cancer.Front Cell Dev Biol. 2021 Apr 27;9:654337. doi: 10.3389/fcell.2021.654337. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33987181 Free PMC article.
-
Shrimp Glucose-6-phosphatase 2 (G6Pase 2): a second isoform of G6Pase in the Pacific white shrimp and regulation of G6Pase 1 and 2 isoforms via HIF-1 during hypoxia and reoxygenation in juveniles.J Bioenerg Biomembr. 2023 Apr;55(2):137-150. doi: 10.1007/s10863-023-09960-z. Epub 2023 Feb 28. J Bioenerg Biomembr. 2023. PMID: 36853470
References
-
- Tso CL, Shintaku P, Chen J, et al. Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res. 2006;4:607–619. - PubMed
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Horwitz EM, Le Blanc K, Dominici M, et al. The International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. - PubMed
-
- Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

