Redox control of the DNA damage-inducible protein DinG helicase activity via its iron-sulfur cluster
- PMID: 19074432
- PMCID: PMC2643519
- DOI: 10.1074/jbc.M807943200
Redox control of the DNA damage-inducible protein DinG helicase activity via its iron-sulfur cluster
Abstract
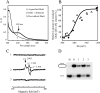
The Escherichia coli DNA damage-inducible protein DinG, a member of the superfamily 2 DNA helicases, has been implicated in the nucleotide excision repair and recombinational DNA repair pathways. Combining UV-visible absorption, EPR, and enzyme activity measurements, we demonstrate here that E. coli DinG contains a redox-active [4Fe-4S] cluster with a midpoint redox potential (E(m)) of -390 +/- 23 mV (pH 8.0) and that reduction of the [4Fe-4S] cluster reversibly switches off the DinG helicase activity. Unlike the [4Fe-4S] cluster in E. coli dihydroxyacid dehydratase, the DinG [4Fe-4S] cluster is stable, and the enzyme remains fully active after exposure to 100-fold excess of hydrogen peroxide, indicating that DinG could be functional under oxidative stress conditions. However, the DinG [4Fe-4S] cluster can be efficiently modified by nitric oxide (NO), forming the DinG-bound dinitrosyl iron complex with the concomitant inactivation of helicase activity in vitro and in vivo. Reassembly of the [4Fe-4S] cluster in NO-modified DinG restores helicase activity, indicating that the iron-sulfur cluster in DinG is the primary target of NO cytotoxicity. The results led us to propose that the iron-sulfur cluster in DinG may act as a sensor of intracellular redox potential to modulate its helicase activity and that modification of the iron-sulfur cluster in DinG and likely in other DNA repair enzymes by NO may contribute to NO-mediated genomic instability.
Figures





Similar articles
-
Reversible inactivation of E. coli endonuclease III via modification of its [4Fe-4S] cluster by nitric oxide.DNA Repair (Amst). 2003 Jul 16;2(7):809-17. doi: 10.1016/s1568-7864(03)00065-x. DNA Repair (Amst). 2003. PMID: 12826281
-
Reactivity of nitric oxide with the [4Fe-4S] cluster of dihydroxyacid dehydratase from Escherichia coli.Biochem J. 2009 Feb 1;417(3):783-9. doi: 10.1042/BJ20081423. Biochem J. 2009. PMID: 18945212 Free PMC article.
-
IscA/SufA paralogues are required for the [4Fe-4S] cluster assembly in enzymes of multiple physiological pathways in Escherichia coli under aerobic growth conditions.Biochem J. 2009 May 27;420(3):463-72. doi: 10.1042/BJ20090206. Biochem J. 2009. PMID: 19309314 Free PMC article.
-
Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide.Acc Chem Res. 2014 Oct 21;47(10):3196-205. doi: 10.1021/ar5002507. Epub 2014 Sep 29. Acc Chem Res. 2014. PMID: 25262769 Review.
-
Reactions of nitric oxide and oxygen with the regulator of fumarate and nitrate reduction, a global transcriptional regulator, during anaerobic growth of Escherichia coli.Methods Enzymol. 2008;437:191-209. doi: 10.1016/S0076-6879(07)37011-0. Methods Enzymol. 2008. PMID: 18433630 Review.
Cited by
-
Genetic analysis of Escherichia coli RadA: functional motifs and genetic interactions.Mol Microbiol. 2015 Mar;95(5):769-79. doi: 10.1111/mmi.12899. Epub 2015 Jan 30. Mol Microbiol. 2015. PMID: 25484163 Free PMC article.
-
DNA Charge Transport: from Chemical Principles to the Cell.Cell Chem Biol. 2016 Jan 21;23(1):183-197. doi: 10.1016/j.chembiol.2015.11.010. Cell Chem Biol. 2016. PMID: 26933744 Free PMC article. Review.
-
Redox Signaling through DNA.Isr J Chem. 2016 Oct;56(9-10):705-723. doi: 10.1002/ijch.201600022. Epub 2016 Jul 29. Isr J Chem. 2016. PMID: 28090121 Free PMC article.
-
8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress.Free Radic Biol Med. 2010 Aug 15;49(4):587-96. doi: 10.1016/j.freeradbiomed.2010.05.008. Epub 2010 May 17. Free Radic Biol Med. 2010. PMID: 20483371 Free PMC article.
-
Positioning Diverse Type IV Structures and Functions Within Class 1 CRISPR-Cas Systems.Front Microbiol. 2021 May 21;12:671522. doi: 10.3389/fmicb.2021.671522. eCollection 2021. Front Microbiol. 2021. PMID: 34093491 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

