Functional rescue of mutant human cystathionine beta-synthase by manipulation of Hsp26 and Hsp70 levels in Saccharomyces cerevisiae
- PMID: 19074437
- PMCID: PMC2640983
- DOI: 10.1074/jbc.M806387200
Functional rescue of mutant human cystathionine beta-synthase by manipulation of Hsp26 and Hsp70 levels in Saccharomyces cerevisiae
Abstract
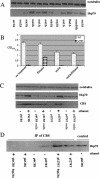
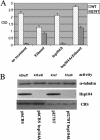
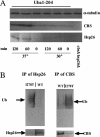
Many human diseases are caused by missense substitutions that result in misfolded proteins that lack biological function. Here we express a mutant form of the human cystathionine beta-synthase protein, I278T, in Saccharomyces cerevisiae and show that it is possible to dramatically restore protein stability and enzymatic function by manipulation of the cellular chaperone environment. We demonstrate that Hsp70 and Hsp26 bind specifically to I278T but that these chaperones have opposite biological effects. Ethanol treatment induces Hsp70 and causes increased activity and steady-state levels of I278T. Deletion of the SSA2 gene, which encodes a cytoplasmic isoform of Hsp70, eliminates the ability of ethanol to restore function, indicating that Hsp70 plays a positive role in proper I278T folding. In contrast, deletion of HSP26 results in increased I278T protein and activity, whereas overexpression of Hsp26 results in reduced I278T protein. The Hsp26-I278T complex is degraded via a ubiquitin/proteosome-dependent mechanism. Based on these results we propose a novel model in which the ratio of Hsp70 and Hsp26 determines whether misfolded proteins will either be refolded or degraded.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

