A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis
- PMID: 19074442
- PMCID: PMC2640977
- DOI: 10.1074/jbc.M807665200
A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis
Abstract
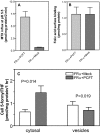
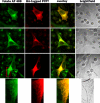
Recently, this laboratory identified a proton-coupled folate transporter (PCFT), with optimal activity at low pH. PCFT is critical to intestinal folate absorption and transport into the central nervous system because there are loss-of-function mutations in this gene in the autosomal recessive disorder, hereditary folate malabsorption. The current study addresses the role PCFT might play in another transport pathway, folate receptor (FR)-mediated endocytosis. FRalpha cDNA was transfected into novel PCFT(+) and PCFT(-) HeLa sublines. FRalpha was shown to bind and trap folates in vesicles but with minimal export into the cytosol in PCFT(-) cells. Cotransfection of FRalpha and PCFT resulted in enhanced folate transport into cytosol as compared with transfection of FRalpha alone. Probenecid did not inhibit folate binding to FR, but inhibited PCFT-mediated transport at endosomal pH, and blocked FRalpha-mediated transport into the cytosol. FRalpha and PCFT co-localized to the endosomal compartment. These observations (i) indicate that PCFT plays a role in FRalpha-mediated endocytosis by serving as a route of export of folates from acidified endosomes and (ii) provide a functional role for PCFT in tissues in which it is expressed, such as the choroid plexus, where the extracellular milieu is at neutral pH.
Figures





Similar articles
-
The proton-coupled folate transporter (PCFT-SLC46A1) and the syndrome of systemic and cerebral folate deficiency of infancy: Hereditary folate malabsorption.Mol Aspects Med. 2017 Feb;53:57-72. doi: 10.1016/j.mam.2016.09.002. Epub 2016 Sep 21. Mol Aspects Med. 2017. PMID: 27664775 Free PMC article. Review.
-
Functional roles of the A335 and G338 residues of the proton-coupled folate transporter (PCFT-SLC46A1) mutated in hereditary folate malabsorption.Am J Physiol Cell Physiol. 2012 Oct 15;303(8):C834-42. doi: 10.1152/ajpcell.00171.2012. Epub 2012 Jul 25. Am J Physiol Cell Physiol. 2012. PMID: 22843796 Free PMC article.
-
A novel loss-of-function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function.Blood. 2008 Sep 1;112(5):2055-61. doi: 10.1182/blood-2008-04-150276. Epub 2008 Jun 17. Blood. 2008. PMID: 18559978
-
Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model.J Neurochem. 2008 Mar;104(6):1494-503. doi: 10.1111/j.1471-4159.2007.05095.x. Epub 2007 Dec 10. J Neurochem. 2008. PMID: 18086128
-
The intestinal absorption of folates.Annu Rev Physiol. 2014;76:251-74. doi: 10.1146/annurev-physiol-020911-153251. Annu Rev Physiol. 2014. PMID: 24512081 Free PMC article. Review.
Cited by
-
A novel antifolate suppresses growth of FPGS-deficient cells and overcomes methotrexate resistance.Life Sci Alliance. 2023 Aug 17;6(11):e202302058. doi: 10.26508/lsa.202302058. Print 2023 Nov. Life Sci Alliance. 2023. PMID: 37591722 Free PMC article.
-
The nexus of vitamin homeostasis and DNA synthesis and modification in mammalian brain.Mol Brain. 2014 Jan 10;7:3. doi: 10.1186/1756-6606-7-3. Mol Brain. 2014. PMID: 24410751 Free PMC article. Review.
-
Mechanistic Target of Rapamycin Is a Novel Molecular Mechanism Linking Folate Availability and Cell Function.J Nutr. 2017 Jul;147(7):1237-1242. doi: 10.3945/jn.117.248823. Epub 2017 Jun 7. J Nutr. 2017. PMID: 28592519 Free PMC article. Review.
-
Enhanced receptor-mediated endocytosis and cytotoxicity of a folic acid-desacetylvinblastine monohydrazide conjugate in a pemetrexed-resistant cell line lacking folate-specific facilitative carriers but with increased folate receptor expression.Mol Pharmacol. 2014 Feb;85(2):310-21. doi: 10.1124/mol.113.089110. Epub 2013 Nov 18. Mol Pharmacol. 2014. PMID: 24249723 Free PMC article.
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
References
-
- Qiu, A., Jansen, M., Sakaris, A., Min, S. H., Chattopadhyay, S., Tsai, E., Sandoval, C., Zhao, R., Akabas, M. H., and Goldman, I. D. (2006) Cell 127 917–928 - PubMed
-
- Lasry, I., Berman, B., Straussberg, R., Sofer, Y., Bessler, H., Sharkia, M., Glaser, F., Jansen, G., Drori, S., and Assaraf, Y. G. (2008) Blood 112 2055–2061 - PubMed
-
- Geller, J., Kronn, D., Jayabose, S., and Sandoval, C. (2002) Medicine (Baltimore) 81 51–68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

