Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation
- PMID: 19074476
- PMCID: PMC4427027
- DOI: 10.1161/CIRCRESAHA.108.190397
Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation
Abstract
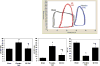
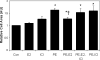
Left ventricular (LV) hypertrophy commonly develops in response to chronic hypertension and is a significant risk factor for heart failure and death. The serine-threonine phosphatase calcineurin (Cn)A plays a critical role in the development of pathological hypertrophy. Previous experimental studies in murine models show that estrogen limits pressure overload-induced hypertrophy; our purpose was to explore further the mechanisms underlying this estrogen effect. Wild-type, ovariectomized female mice were treated with placebo or 17beta-estradiol (E2), followed by transverse aortic constriction (TAC), to induce pressure overload. At 2 weeks, mice underwent physiological evaluation, immediate tissue harvest, or dispersion of cardiomyocytes. E2 replacement limited TAC-induced LV and cardiomyocyte hypertrophy while attenuating deterioration in LV systolic function and contractility. These E2 effects were associated with reduced abundance of CnA. The primary downstream targets of CnA are the nuclear factor of activated T-cell (NFAT) family of transcription factors. In transgenic mice expressing a NFAT-activated promoter/luciferase reporter gene, E2 limited TAC-induced activation of NFAT. Moreover, the inhibitory effects of E2 on LV hypertrophy were absent in CnA knockout mice, supporting the notion that CnA is an important target of E2-mediated inhibition. In cultured rat cardiac myocytes, E2 inhibited agonist-induced hypertrophy while also decreasing CnA abundance and NFAT activation. Agonist stimulation also reduced CnA ubiquitination and degradation that was prevented by E2; all in vitro effects of estrogen were reversed by an estrogen receptor (ER) antagonist. These data support that E2 reduces pressure overload induced hypertrophy by an ER-dependent mechanism that increases CnA degradation, unveiling a novel mechanism by which E2 and ERs regulate pathological LV and cardiomyocyte growth.
Figures





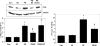
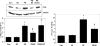
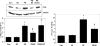
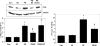





References
-
- American Heart Association. Heart Disease and Stroke Statistics: 2005 Statistical Supplement. Dallas, TX: American Heart Association; 2005.
-
- Patten RD, Udelson JE, Konstam MA. LV dysfunction and Heart Failure Following Myocardial Infarction. In: Sharpe N, editor. Heart Failure Management. London: Martin Duntz LTD; 2000. pp. 183–198.
-
- Molkentin JD, Dorn GW., II Cytoplasmic Signaling Pathways That Regulate Cardiac Hypertrophy. Annual Review of Physiology. 2001;63:391–426. - PubMed
-
- Patten RD. Cellular, Molecular and Structural Changes During Cardiac Remodeling. In: Hosenpud J, Greenberg B, editors. Congestive Heart Failure. 3. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007. pp. 128–146.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

