Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation
- PMID: 19074510
- PMCID: PMC2660643
- DOI: 10.1096/fj.08-120006
Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation
Abstract
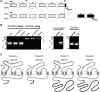
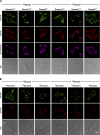
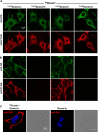
Dual oxidases (Duox1 and Duox2) are plasma membrane-targeted hydrogen peroxide generators that support extracellular hemoperoxidases. Duox activator 2 (Duoxa2), initially described as an endoplasmic reticulum resident protein, functions as a maturation factor needed to deliver active Duox2 to the cell surface. However, less is known about the Duox1/Duoxa1 homologues. We identified four alternatively spliced Duoxa1 variants and explored their roles in Duox subcellular targeting and reconstitution. Duox1 and Duox2 are functionally rescued by Duoxa2 or the Duoxa1 variants that contain the third coding exon. All active maturation factors are cotransported to the cell surface when coexpressed with either Duox1 or Duox2, consistent with detection of endogenous Duoxa1 on apical plasma membranes of the airway epithelium. In contrast, the Duoxa proteins are retained in the endoplasmic reticulum when expressed without Duox. Duox1/Duoxa1alpha and Duox2/Duoxa2 pairs produce the highest levels of hydrogen peroxide, as they undergo Golgi-based carbohydrate modifications and form stable cell surface complexes. Cross-functioning pairs that do not form stable complexes produce less hydrogen peroxide and leak superoxide. These findings suggest Duox activators not only promote Duox maturation, but they function as part of the hydrogen peroxide-generating enzyme.
Figures






Similar articles
-
The Dual Oxidase Duox2 stabilized with DuoxA2 in an enzymatic complex at the surface of the cell produces extracellular H2O2 able to induce DNA damage in an inducible cellular model.Exp Cell Res. 2019 Nov 1;384(1):111620. doi: 10.1016/j.yexcr.2019.111620. Epub 2019 Sep 9. Exp Cell Res. 2019. PMID: 31513783
-
The NADPH oxidases DUOX1 and DUOX2 are sorted to the apical plasma membrane in epithelial cells via their respective maturation factors DUOXA1 and DUOXA2.Genes Cells. 2024 Oct;29(10):921-930. doi: 10.1111/gtc.13153. Epub 2024 Aug 10. Genes Cells. 2024. PMID: 39126279 Free PMC article.
-
The extracellular A-loop of dual oxidases affects the specificity of reactive oxygen species release.J Biol Chem. 2015 Mar 6;290(10):6495-506. doi: 10.1074/jbc.M114.592717. Epub 2015 Jan 13. J Biol Chem. 2015. PMID: 25586178 Free PMC article.
-
Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases.Antioxid Redox Signal. 2009 Oct;11(10):2607-19. doi: 10.1089/ars.2009.2637. Antioxid Redox Signal. 2009. PMID: 19438290 Free PMC article. Review.
-
Dual oxidase, hydrogen peroxide and thyroid diseases.Exp Biol Med (Maywood). 2010 Apr;235(4):424-33. doi: 10.1258/ebm.2009.009241. Exp Biol Med (Maywood). 2010. PMID: 20407074 Review.
Cited by
-
Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans.Infect Immun. 2009 Nov;77(11):4983-9. doi: 10.1128/IAI.00627-09. Epub 2009 Aug 17. Infect Immun. 2009. PMID: 19687201 Free PMC article.
-
Mammalian numb-interacting protein 1/dual oxidase maturation factor 1 directs neuronal fate in stem cells.J Biol Chem. 2010 Jun 4;285(23):17974-85. doi: 10.1074/jbc.M109.084616. Epub 2010 Mar 16. J Biol Chem. 2010. PMID: 20233719 Free PMC article.
-
Persistent goiter with congenital hypothyroidism due to mutation in DUOXA2 gene.Ann Pediatr Endocrinol Metab. 2020 Mar;25(1):57-62. doi: 10.6065/apem.2020.25.1.57. Epub 2020 Mar 31. Ann Pediatr Endocrinol Metab. 2020. PMID: 32252219 Free PMC article.
-
The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy.Int J Mol Sci. 2021 Jan 5;22(1):462. doi: 10.3390/ijms22010462. Int J Mol Sci. 2021. PMID: 33466458 Free PMC article.
-
DUOX2/DUOXA2 Mutations Frequently Cause Congenital Hypothyroidism that Evades Detection on Newborn Screening in the United Kingdom.Thyroid. 2019 Jun;29(6):790-801. doi: 10.1089/thy.2018.0587. Thyroid. 2019. PMID: 31044655 Free PMC article.
References
-
- Dupuy C, Ohayon R, Valent A, Noel-Hudson M S, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. - PubMed
-
- De Deken X, Wang D, Many M C, Costagliola S, Libert F, Vassart G, Dumont J E, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. - PubMed
-
- Moreno J C, Bikker H, Kempers M J, van Trotsenburg A S, Baas F, de Vijlder J J, Vulsma T, Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002;347:95–102. - PubMed
-
- Vigone M C, Fugazzola L, Zamproni I, Passoni A, Di Candia S, Chiumello G, Persani L, Weber G. Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum Mutat. 2005;26:395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

