Long-loop pathways in cardiovascular electroacupuncture responses
- PMID: 19074569
- PMCID: PMC2644252
- DOI: 10.1152/japplphysiol.91277.2008
Long-loop pathways in cardiovascular electroacupuncture responses
Abstract
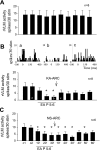
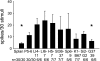
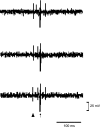
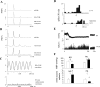
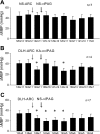
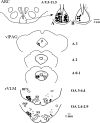
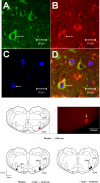
We have shown that electroacupuncture (EA) at P 5-6 (overlying median nerves) activates arcuate (ARC) neurons, which excite the ventrolateral periaqueductal gray (vlPAG) and inhibit cardiovascular sympathoexcitatory neurons in the rostral ventrolateral medulla (rVLM). To investigate whether the ARC inhibits rVLM activity directly or indirectly, we stimulated the splanchnic nerve to activate rVLM neurons. Micropipettes were inserted in the rVLM, vlPAG, and ARC for neural recording or injection. Microinjection of kainic acid (KA; 1 mM, 50 nl) in the ARC blocked EA inhibition of the splanchnic nerve stimulation-induced reflex increases in rVLM neuronal activity. Microinjection of d,l-homocysteic acid (4 nM, 50 nl) in the ARC, like EA, inhibited reflex increases in the rVLM neuronal discharge. The vlPAG neurons receive convergent input from the ARC, splanchnic nerve, P 5-6, and other acupoints. Microinjection of KA bilaterally into the rostral vlPAG partially reversed rVLM neuronal responses and cardiovascular inhibition during d,l-homocysteic acid stimulation of the ARC. On the other hand, injection of KA into the caudal vlPAG completely reversed these responses. We also observed that ARC neurons could be antidromically activated by stimulating the rVLM, and that ARC perikarya was labeled with retrograde tracer that had been microinjected into the rVLM. These neurons frequently contained beta-endorphin and c-Fos, activated by EA stimulation. Therefore, the vlPAG, particularly, the caudal vlPAG, is required for ARC inhibition of rVLM neuronal activation and subsequent EA-related cardiovascular activation. Direct projections from the ARC to the rVLM, which serve as an important source of beta-endorphin, appear also to exist.
Figures

References
-
- Barman S, Gebber G. Sequence of activation of ventrolateral and dorsal medullary sympathetic neurons. Am J Physiol Regul Integr Comp Physiol 245: R438–R447, 1983. - PubMed
-
- Berman AL The Brainstem of the Cat: A Cytoarchitectonic Atlas With Stereotaxic Coordinates. Madison, WI: University of Wisconsin Press, 1968.
-
- Bureš J, Petran M, Zachar J. Electrophysiological Methods in Biological Research. New York: Academic, 1967.
-
- Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal: a correlative functional and anatomical study. Brain Res 541: 206–216, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

