Thyroid hormone receptor-beta is associated with coronary angiogenesis during pathological cardiac hypertrophy
- PMID: 19074585
- PMCID: PMC2659277
- DOI: 10.1210/en.2008-0634
Thyroid hormone receptor-beta is associated with coronary angiogenesis during pathological cardiac hypertrophy
Abstract
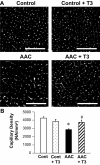
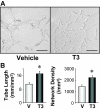
Insufficient angiogenesis is one of the causes leading to tissue ischemia and dysfunction. In heart failure, there is increasing evidence showing decreased capillary density in the left ventricle (LV) myocardium, although the detailed mechanisms contributing to it are not clear. The goal of this study was to investigate the role of thyroid hormone receptors (TRs) in the coronary microvascular rarefaction under pathological cardiac hypertrophy. The LV from hypertrophied/failing hearts induced by ascending aortic constriction (AAC) exhibited severe microvascular rarefaction, and this phenomenon was restored by chronic T(3) administration. Coronary endothelial cells (ECs) isolated from AAC hearts expressed lower TRbeta mRNA than control ECs, and chronic T(3) administration restored TRbeta mRNA expression level in AAC hearts to the control level. Among different TR subtype-specific knockout mice, TRbeta knockout and TRalpha/TRbeta double-knockout mice both exhibited significantly less capillary density in LV compared with wild-type mice. In vitro, coronary ECs isolated from TRbeta knockout mice lacked the ability to form capillary networks. In addition, we identified that kinase insert domain protein receptor/fetal liver kinase-1 (vascular endothelial growth factor-2 receptor) was one of the angiogenic mediators controlled by T(3) administration in the AAC heart. These data suggest that TRbeta in the coronary ECs regulates capillary density during cardiac development, and down-regulation of TRbeta results in coronary microvascular rarefaction during pathological hypertrophy.
Figures


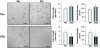

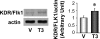

Similar articles
-
Regulatory role of TIGAR on endothelial metabolism and angiogenesis.J Cell Physiol. 2021 Nov;236(11):7578-7590. doi: 10.1002/jcp.30401. Epub 2021 Apr 30. J Cell Physiol. 2021. PMID: 33928637 Free PMC article.
-
Thyroid hormone induces sprouting angiogenesis in adult heart of hypothyroid mice through the PDGF-Akt pathway.J Cell Mol Med. 2012 Nov;16(11):2726-35. doi: 10.1111/j.1582-4934.2012.01593.x. J Cell Mol Med. 2012. PMID: 22681587 Free PMC article.
-
Both thyroid hormone receptor (TR)beta 1 and TR beta 2 isoforms contribute to the regulation of hypothalamic thyrotropin-releasing hormone.Endocrinology. 2004 May;145(5):2337-45. doi: 10.1210/en.2003-1209. Epub 2004 Jan 15. Endocrinology. 2004. PMID: 14726446
-
Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction.Am J Physiol Heart Circ Physiol. 2014 Dec 15;307(12):H1691-704. doi: 10.1152/ajpheart.00281.2014. Epub 2014 Sep 19. Am J Physiol Heart Circ Physiol. 2014. PMID: 25239805 Free PMC article.
-
Therapeutic potential for thyroid hormone receptor-beta selective agonists for treating obesity, hyperlipidemia and diabetes.Curr Vasc Pharmacol. 2007 Apr;5(2):141-54. doi: 10.2174/157016107780368271. Curr Vasc Pharmacol. 2007. PMID: 17430219 Review.
Cited by
-
The Role of Thyroid Diseases and their Medications in Cardiovascular Disorders: A Review of the Literature.Curr Cardiol Rev. 2020;16(2):103-116. doi: 10.2174/1573403X15666191008111238. Curr Cardiol Rev. 2020. PMID: 31593532 Free PMC article. Review.
-
Deciphering direct and indirect influence of thyroid hormone with mouse genetics.Mol Endocrinol. 2014 Apr;28(4):429-41. doi: 10.1210/me.2013-1414. Epub 2014 Mar 10. Mol Endocrinol. 2014. PMID: 24617548 Free PMC article. Review.
-
Low-dose T₃ replacement restores depressed cardiac T₃ levels, preserves coronary microvasculature and attenuates cardiac dysfunction in experimental diabetes mellitus.Mol Med. 2014 May 1;20(1):302-12. doi: 10.2119/molmed.2013.00040. Mol Med. 2014. PMID: 24960246 Free PMC article.
-
Role of the Thyroid System in the Dynamic Complex Network of Cardioprotection.Eur Cardiol. 2016 Aug;11(1):36-42. doi: 10.15420/ecr.2016:9:2. Eur Cardiol. 2016. PMID: 30310446 Free PMC article. Review.
-
VDAC: old protein with new roles in diabetes.Am J Physiol Cell Physiol. 2012 Nov 15;303(10):C1055-60. doi: 10.1152/ajpcell.00087.2012. Epub 2012 Sep 12. Am J Physiol Cell Physiol. 2012. PMID: 22972802 Free PMC article.
References
-
- Yla-Herttuala S, Martin JF 2000 Cardiovascular gene therapy. Lancet 355:213–222 - PubMed
-
- Tamirisa KP, Mukherjee D 2002 Gene therapy in cardiovascular diseases. Curr Gene Ther 2:427–435 - PubMed
-
- Mukherjee D 2004 Current clinical perspectives on myocardial angiogenesis. Mol Cell Biochem 264:157–167 - PubMed
-
- Klein I, Ojamaa K 2001 Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 - PubMed
-
- Motomura K, Brent GA 1998 Mechanisms of thyroid hormone action. Implications for the clinical manifestation of thyrotoxicosis. Endocrinol Metab Clin North Am 27:1–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

