Functional differentiation of tbf1 orthologues in fission and budding yeasts
- PMID: 19074598
- PMCID: PMC2643609
- DOI: 10.1128/EC.00174-08
Functional differentiation of tbf1 orthologues in fission and budding yeasts
Abstract
In Saccharomyces cerevisiae, TBF1, an essential gene, influences telomere function but also has other roles in the global regulation of transcription. We have identified a new member of the tbf1 gene family in the mammalian pathogen Pneumocystis carinii. We demonstrate by transspecies complementation that its ectopic expression can provide the essential functions of Schizosaccharomyces pombe tbf1 but that there is no rescue between fission and budding yeast orthologues. Our findings indicate that an essential function of this family of proteins has diverged in the budding and fission yeasts and suggest that effects on telomere length or structure are not the primary cause of inviability in S. pombe tbf1 null strains.
Figures


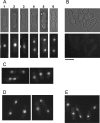
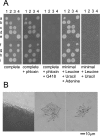
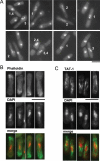
Similar articles
-
Involvement of rhp23, a Schizosaccharomyces pombe homolog of the human HHR23A and Saccharomyces cerevisiae RAD23 nucleotide excision repair genes, in cell cycle control and protein ubiquitination.Nucleic Acids Res. 2002 Jan 15;30(2):581-91. doi: 10.1093/nar/30.2.581. Nucleic Acids Res. 2002. PMID: 11788722 Free PMC article.
-
Structural and functional insights into yeast Tbf1 as an atypical telomeric repeat-binding factor.Structure. 2024 Jul 11;32(7):889-898.e3. doi: 10.1016/j.str.2024.04.002. Epub 2024 Apr 26. Structure. 2024. PMID: 38677290
-
Identification and characterization of an essential telomeric repeat binding factor in fission yeast.J Biol Chem. 2008 Feb 1;283(5):2693-701. doi: 10.1074/jbc.M708784200. Epub 2007 Oct 30. J Biol Chem. 2008. PMID: 17977837
-
Genetic approaches to aging in budding and fission yeasts: new connections and new opportunities.Subcell Biochem. 2012;57:291-314. doi: 10.1007/978-94-007-2561-4_13. Subcell Biochem. 2012. PMID: 22094427 Review.
-
A conserved role of the RSC chromatin remodeler in the establishment of nucleosome-depleted regions.Curr Genet. 2017 May;63(2):187-193. doi: 10.1007/s00294-016-0642-y. Epub 2016 Aug 24. Curr Genet. 2017. PMID: 27558480 Free PMC article. Review.
Cited by
-
Tbf1 and Vid22 promote resection and non-homologous end joining of DNA double-strand break ends.EMBO J. 2013 Jan 23;32(2):275-89. doi: 10.1038/emboj.2012.327. Epub 2012 Dec 7. EMBO J. 2013. PMID: 23222485 Free PMC article.
-
Functional characterization of the Pneumocystis jirovecii potential drug targets dhfs and abz2 involved in folate biosynthesis.Antimicrob Agents Chemother. 2015 May;59(5):2560-6. doi: 10.1128/AAC.05092-14. Epub 2015 Feb 17. Antimicrob Agents Chemother. 2015. PMID: 25691634 Free PMC article.
-
Subtelomere-binding protein Tbf1 and telomere-binding protein Rap1 collaborate to inhibit localization of the Mre11 complex to DNA ends in budding yeast.Mol Biol Cell. 2012 Jan;23(2):347-59. doi: 10.1091/mbc.E11-06-0568. Epub 2011 Nov 30. Mol Biol Cell. 2012. PMID: 22130795 Free PMC article.
-
Evidence for Proinflammatory β-1,6 Glucans in the Pneumocystis carinii Cell Wall.Infect Immun. 2015 Jul;83(7):2816-26. doi: 10.1128/IAI.00196-15. Epub 2015 Apr 27. Infect Immun. 2015. PMID: 25916991 Free PMC article.
-
Tay1 protein, a novel telomere binding factor from Yarrowia lipolytica.J Biol Chem. 2010 Dec 3;285(49):38078-92. doi: 10.1074/jbc.M110.127605. Epub 2010 Oct 5. J Biol Chem. 2010. PMID: 20923774 Free PMC article.
References
-
- Ambrose, H. E., S. P. Keely, E. M. Aliouat, E. Dei-Cas, A. E. Wakefield, R. F. Miller, and J. R. Stringer. 2004. Expression and complexity of the PRT1 multigene family of Pneumocystis carinii. Microbiology 150293-300. - PubMed
-
- Aslett, M., and V. Wood. 2006. Gene Ontology annotation status of the fission yeast genome: preliminary coverage approaches 100%. Yeast 23913-919. - PubMed
-
- Barry, J. D., M. L. Ginger, P. Burton, and R. McCulloch. 2003. Why are parasite contingency genes often associated with telomeres? Int. J. Parasitol. 3329-45. - PubMed
-
- Basi, G., E. Schmid, and K. Maundrell. 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123131-136. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

