The role of incretins in glucose homeostasis and diabetes treatment
- PMID: 19074620
- PMCID: PMC2696340
- DOI: 10.1124/pr.108.000604
The role of incretins in glucose homeostasis and diabetes treatment
Abstract
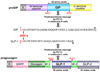
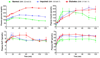
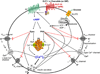
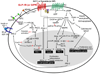
Incretins are gut hormones that are secreted from enteroendocrine cells into the blood within minutes after eating. One of their many physiological roles is to regulate the amount of insulin that is secreted after eating. In this manner, as well as others to be described in this review, their final common raison d'être is to aid in disposal of the products of digestion. There are two incretins, known as glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1), that share many common actions in the pancreas but have distinct actions outside of the pancreas. Both incretins are rapidly deactivated by an enzyme called dipeptidyl peptidase 4 (DPP4). A lack of secretion of incretins or an increase in their clearance are not pathogenic factors in diabetes. However, in type 2 diabetes (T2DM), GIP no longer modulates glucose-dependent insulin secretion, even at supraphysiological (pharmacological) plasma levels, and therefore GIP incompetence is detrimental to beta-cell function, especially after eating. GLP-1, on the other hand, is still insulinotropic in T2DM, and this has led to the development of compounds that activate the GLP-1 receptor with a view to improving insulin secretion. Since 2005, two new classes of drugs based on incretin action have been approved for lowering blood glucose levels in T2DM: an incretin mimetic (exenatide, which is a potent long-acting agonist of the GLP-1 receptor) and an incretin enhancer (sitagliptin, which is a DPP4 inhibitor). Exenatide is injected subcutaneously twice daily and its use leads to lower blood glucose and higher insulin levels, especially in the fed state. There is glucose-dependency to its insulin secretory capacity, making it unlikely to cause low blood sugars (hypoglycemia). DPP4 inhibitors are orally active and they increase endogenous blood levels of active incretins, thus leading to prolonged incretin action. The elevated levels of GLP-1 are thought to be the mechanism underlying their blood glucose-lowering effects.
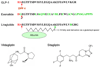
Figures


References
-
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. - PubMed
-
- Acitores A, González N, Sancho V, Valverde I, Villanueva-Peñacarrillo ML. Cell signalling of glucagon-like peptide-1 action in rat skeletal muscle. J Endocrinol. 2004;180:389–398. - PubMed
-
- Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. - PubMed
-
- Ahrén B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am J Physiol Regul Integr Comp Physiol. 2004;286:R269–F272. - PubMed
-
- Alcántara AI, Morales M, Delgado E, López-Delgado MI, Clemente F, Luque MA, Malaisse WJ, Valverde I, Villanueva-Peñacarrillo ML. Exendin-4 agonist and exendin(9–39)amide antagonist of the GLP-1(7–36)amide effects in liver and muscle. Arch Biochem Biophys. 1997;341:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

