Gastrin activates paracrine networks leading to induction of PAI-2 via MAZ and ASC-1
- PMID: 19074642
- PMCID: PMC2643906
- DOI: 10.1152/ajpgi.90340.2008
Gastrin activates paracrine networks leading to induction of PAI-2 via MAZ and ASC-1
Abstract
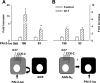
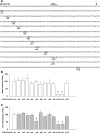
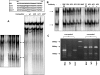
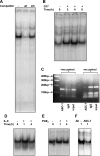
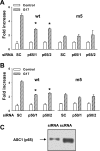
The gastric hormone gastrin regulates the expression of a variety of genes involved in control of acid secretion and also in the growth and organization of the gastric mucosa. One putative target is plasminogen activator inhibitor-2 (PAI-2), which is a component of the urokinase activator system that acts extracellularly to inhibit urokinase plasminogen activator (uPA) and intracellularly to suppress apoptosis. Previous studies have demonstrated that gastrin induces PAI-2 both in gastric epithelial cells expressing the gastrin (CCK-2) receptor and, via activation of paracrine networks, in adjacent cells that do not express the receptor. We have now sought to identify the response element(s) in the PAI-2 promoter targeted by paracrine mediators initiated by gastrin. Mutational analysis identified two putative response elements in the PAI-2 promoter that were downstream of gastrin-activated paracrine signals. One was identified as a putative MAZ site, mutation of which dramatically reduced both basal and gastrin-stimulated responses of the PAI-2 promoter by a mechanism involving PGE(2) and the small GTPase RhoA. Yeast one-hybrid screening identified the other as binding the activating signal cointegrator-1 (ASC-1) complex, which was shown to be the target of IL-8 released by gastrin. RNA interference (RNAi) knockdown of two subunits of the ASC-1 complex (p50 and p65) inhibited induction of PAI-2 expression by gastrin. The data reveal previously unsuspected transcriptional mechanisms activated as a consequence of gastrin-triggered paracrine networks and emphasize the elaborate and complex cellular control mechanisms required for a key component of tissue responses to damage and infection.
Figures

References
-
- Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72: 1–22, 1997. - PubMed
-
- Ansorge N, Juttner S, Cramer T, Schmidt WE, Hocker M, Schmitz F. An upstream CRE-E-box element is essential for gastrin-dependent activation of the cyclooxygenase-2 gene in human colon cancer cells. Regul Pept 144: 25–33, 2007. - PubMed
-
- Antalis TM, Costelloe E, Muddiman J, Ogbourne S, Donnan K. Regulation of the plasminogen activator inhibitor type-2 gene in monocytes: localization of an upstream transcriptional silencer. Blood 88: 3686–3697, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

