Roles of beta-tubulin residues Ala428 and Thr429 in microtubule formation in vivo
- PMID: 19074767
- PMCID: PMC3837401
- DOI: 10.1074/jbc.M807491200
Roles of beta-tubulin residues Ala428 and Thr429 in microtubule formation in vivo
Abstract
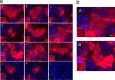
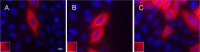
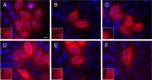
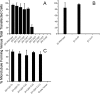
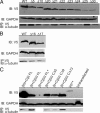
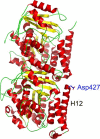
The C termini of beta-tubulin isotypes are regions of high sequence variability that bind to microtubule-associated proteins and motors and undergo various post-translational modifications such as polyglutamylation and polyglycylation. Crystallographic analyses have been unsuccessful in resolving tubulin C termini. Here, we used a stepwise approach to study the role of this region in microtubule assembly. We generated a series of truncation mutants of human betaI and betaIII tubulin. Transient transfection of HeLa cells with the mutants shows that mutants with deletions of up to 22 residues from betaIII and 16 from betaI can assemble normally. Interestingly, removal of the next residue (Ala(428)) results in a complete loss of microtubule formation without affecting dimer formation. C-terminal tail switching of human betaI and betaIII tubulin suggests that C-terminal tails are functionally equivalent. In short, residues outside of 1-429 of human beta-tubulins make no contribution to microtubule assembly. Ala(428), in the C-terminal sequence motif N-QQYQDA(428), lies at the end of helix H12 of beta-tubulin. We hypothesize that this residue is important for maintaining helix H12 structure. Deletion of Ala(428) may lead to unwinding of helix H12, resulting in tubulin dimers incapable of assembly. Thr(429) plays a more complex role. In the betaI isotype of tubulin, Thr(429) is not at all necessary for assembly; however, in the betaIII isotype, its presence strongly favors assembly. This result is consistent with a likely more complex function of betaIII as well as with the observation that evolutionary conservation is total for Ala(428) and frequent for Thr(429).
Figures

Similar articles
-
Novel mutations involving βI-, βIIA-, or βIVB-tubulin isotypes with functional resemblance to βIII-tubulin in breast cancer.Protoplasma. 2017 May;254(3):1163-1173. doi: 10.1007/s00709-016-1060-1. Epub 2016 Dec 9. Protoplasma. 2017. PMID: 27943021
-
Identification of betaIII- and betaIV-tubulin isotypes in cold-adapted microtubules from Atlantic cod (Gadus morhua): antibody mapping and cDNA sequencing.Cell Motil Cytoskeleton. 1999;42(4):315-30. doi: 10.1002/(SICI)1097-0169(1999)42:4<315::AID-CM5>3.0.CO;2-C. Cell Motil Cytoskeleton. 1999. PMID: 10223637
-
Mobility and Core-Protein Binding Patterns of Disordered C-Terminal Tails in β-Tubulin Isotypes.Biochemistry. 2017 Mar 28;56(12):1746-1756. doi: 10.1021/acs.biochem.6b00988. Epub 2017 Mar 17. Biochemistry. 2017. PMID: 28290671
-
[Microtubules: functional polymorphisms of tubulin and associated proteins (structural and motor MAP's)].C R Seances Soc Biol Fil. 1996;190(2-3):255-68. C R Seances Soc Biol Fil. 1996. PMID: 8869236 Review. French.
-
Tubulins as therapeutic targets in cancer: from bench to bedside.Curr Pharm Des. 2012;18(19):2778-92. doi: 10.2174/138161212800626193. Curr Pharm Des. 2012. PMID: 22390762 Review.
Cited by
-
The C terminus of tubulin, a versatile partner for cationic molecules: binding of Tau, polyamines, and calcium.J Biol Chem. 2011 Jan 28;286(4):3065-78. doi: 10.1074/jbc.M110.144089. Epub 2010 Nov 9. J Biol Chem. 2011. PMID: 21062741 Free PMC article.
-
Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: implications for side effects of general anesthesia.PLoS One. 2012;7(6):e37251. doi: 10.1371/journal.pone.0037251. Epub 2012 Jun 25. PLoS One. 2012. PMID: 22761654 Free PMC article.
-
Polyglutamylation: biology and analysis.Amino Acids. 2022 Apr;54(4):529-542. doi: 10.1007/s00726-022-03146-4. Epub 2022 Mar 31. Amino Acids. 2022. PMID: 35357568 Free PMC article. Review.
-
Possible Roles of Specific Amino Acids in β-Tubulin Isotypes in the Growth and Maintenance of Neurons: Novel Insights From Cephalopod Mollusks.Front Mol Neurosci. 2022 Apr 14;15:838393. doi: 10.3389/fnmol.2022.838393. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35493322 Free PMC article.
-
The beta isotypes of tubulin in neuronal differentiation.Cytoskeleton (Hoboken). 2010 Jul;67(7):431-41. doi: 10.1002/cm.20455. Cytoskeleton (Hoboken). 2010. PMID: 20506160 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

