Lipid-protein interactions drive membrane protein topogenesis in accordance with the positive inside rule
- PMID: 19074771
- PMCID: PMC2665083
- DOI: 10.1074/jbc.R800081200
Lipid-protein interactions drive membrane protein topogenesis in accordance with the positive inside rule
Abstract
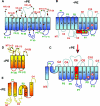
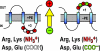
Transmembrane domain orientation within some membrane proteins is dependent on membrane lipid composition. Initial orientation occurs within the translocon, but final orientation is determined after membrane insertion by interactions within the protein and between lipid headgroups and protein extramembrane domains. Positively and negatively charged amino acids in extramembrane domains represent cytoplasmic retention and membrane translocation forces, respectively, which are determinants of protein orientation. Lipids with no net charge dampen the translocation potential of negative residues working in opposition to cytoplasmic retention of positive residues, thus allowing the functional presence of negative residues in cytoplasmic domains without affecting protein topology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

