A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa
- PMID: 19074801
- PMCID: PMC2823395
- DOI: 10.1167/iovs.08-2497
A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa
Abstract
Purpose: Interphotoreceptor retinoid-binding protein (IRBP) has been considered essential for normal rod and cone function, as it mediates the transport of retinoids between the photoreceptors and the retinal pigment epithelium. This study was performed to determine whether mutations in the IRBP gene (RBP3) are associated with photoreceptor degeneration.
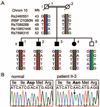
Methods: A consanguineous family was ascertained in which four children had autosomal recessive retinitis pigmentosa (RP). Homozygosity mapping performed with SNP microarrays revealed only one homozygous region shared by all four affected siblings. Sequencing of RBP3, contained in this region, was performed in this family and others with recessive RP. Screening was also performed on patients with various other forms of retinal degeneration or malfunction.
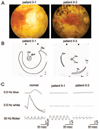
Results: Sequence analysis of RBP3 revealed a homozygous missense mutation (p.Asp1080Asn) in the four affected siblings. The mutation affects a residue that is completely conserved in all four homologous modules of the IRBP protein of vertebrate species and in C-terminal-processing proteases, photosynthesis enzymes found in bacteria, algae, and plants. Based on the previously reported crystal structure of Xenopus IRBP, the authors predict that the Asp1080-mediated conserved salt bridge that appears to participate in scaffolding of the retinol-binding domain is abolished by the mutation. No RBP3 mutations were detected in 395 unrelated patients with recessive or isolate RP or in 680 patients with other forms of hereditary retinal degeneration.
Conclusions: Mutations in RBP3 are an infrequent cause of autosomal recessive RP. The mutation Asp1080Asn may alter the conformation of the IRBP protein by disrupting a conserved salt bridge.
Figures



References
-
- Adler AJ, Martin KJ. Retinol-binding proteins in bovine interphotoreceptor matrix. Biochem Biophys Res Commun. 1982;108:1601–1608. - PubMed
-
- Pfeffer B, Wiggert B, Lee L, Zonnenberg B, Newsome D, Chader G. The presence of a soluble interphotoreceptor retinol-binding protein (IRBP) in the retinal interphotoreceptor space. J Cell Physiol. 1983;117:333–341. - PubMed
-
- Liou GI, Bridges CD, Fong SL, Alvarez RA, Gonzalez-Fernandez F. Vitamin A transport between retina and pigment epithelium-an interstitial protein carrying endogenous retinol (interstitial retinolbinding protein) Vision Res. 1982;22:1457–1467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

