Sublethal photic stress and the motility of RPE phagosomes and melanosomes
- PMID: 19074812
- PMCID: PMC2675274
- DOI: 10.1167/iovs.08-2671
Sublethal photic stress and the motility of RPE phagosomes and melanosomes
Abstract
Purpose: To determine whether sublethal oxidative stress to the retinal pigment epithelium by visible light treatment affects the translocation of organelles, notably phagosomes and melanosomes.
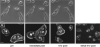
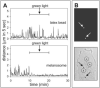
Methods: Isolated porcine melanosomes were phagocytized by ARPE-19 cells, then cultures were treated with blue light to generate reactive oxygen intermediates (ROIs) by endogenous retinal pigment epithelial (RPE) chromophores throughout the cytoplasm. Other melanosomes were preloaded with a photosensitizer before phagocytosis, and cells were light treated to generate ROIs specifically at the granule surface. Phagosome movement was analyzed by live cell imaging. Also analyzed were phagocytized black latex beads, phagocytized melanosomes pretreated to simulate age-related melanin photobleaching, and endogenous RPE melanosomes in primary cultures of porcine retinal pigment epithelium.
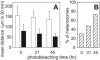
Results: Sublethal blue light treatment slowed the movement of some, but not all, phagocytized melanosomes. All phagosomes slowed when ROIs were generated near the organelles through a photosensitized reaction. Melanosome photobleaching, which makes granules more photoreactive, increased the effects of blue light. Blue light treatment also slowed the motility of phagosomes containing latex beads and endogenous pigment granules.
Conclusions: Blue light-induced stress impairs phagosome motility in RPE cells but affects individual organelles differently, suggesting that the effects of mild oxidative injury vary with subcellular location. The mechanisms underlying slowed motility are at least partially local because slowing can be induced by a photosensitized reaction in the subdomain of the organelle and the magnitude of the slowing is greater when the phagosome contents are photoreactive. Photic stress may impair the movement and positioning of RPE organelles, which would have widespread consequences for maintaining a functionally efficient subcellular organization.
Figures




References
-
- Barral DC, Seabra MC. The melanosome as a model to study organelle motility in mammals. Pigment Cell Res. 2004;17:111–118. - PubMed
-
- Futter CE. The molecular regulation of organelle transport in mammalian retinal pigment epithelial cells. Pigment Cell Res. 2006;19:104–111. - PubMed
-
- Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–399. - PubMed
-
- Aslan M, Ryan TM, Townes TM, et al. Nitric oxide-dependent generation of reactive species in sickle cell disease: actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–4204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

