The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting
- PMID: 19074825
- PMCID: PMC3690928
- DOI: 10.1158/1541-7786.MCR-08-0244
The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting
Abstract
The Eph receptor tyrosine kinases and ephrin ligands have been studied extensively for their roles in developmental processes. In recent years, Eph receptors and ephrins have been found to be integral players in cancer formation and progression. Among these are EphA2 and ephrinA1, which are involved in the development and maintenance of many different types of solid tumors. The function of EphA2 and ephrinA1 in tumorigenesis and tumor progression is complex and seems to be dependent on cell type and microenvironment. These variables affect the expression of the EphA2 and ephrinA1 proteins, the pathways through which they induce signaling, and the functional consequences of that signaling on the behavior of tumor cells and tumor-associated cells. This review will specifically focus on the roles that EphA2 and ephrinA1 play in the different cell types that contribute to the malignancy of solid tumors, with emphasis on the opportunities for therapeutic targeting.
Conflict of interest statement
No potential conflicts of interest were disclosed.
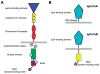
Figures

References
-
- Bartley TD, Hunt RW, Welcher AA, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558– 60. - PubMed
-
- Gale NW, Yancopoulos GD. Ephrins and their receptors: a repulsive topic? Cell Tissue Res. 1997;290:227– 41. - PubMed
-
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717– 20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

