p53 Small-molecule inhibitor enhances temozolomide cytotoxic activity against intracranial glioblastoma xenografts
- PMID: 19074867
- PMCID: PMC2987557
- DOI: 10.1158/0008-5472.CAN-08-1687
p53 Small-molecule inhibitor enhances temozolomide cytotoxic activity against intracranial glioblastoma xenografts
Abstract
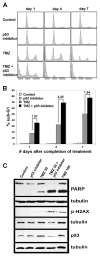
In this study, we investigated the precursor and active forms of a p53 small-molecule inhibitor for their effects on temozolomide (TMZ) antitumor activity against glioblastoma (GBM), using both in vitro and in vivo experimental approaches. Results from in vitro cell viability analysis showed that the cytotoxic activity of TMZ was substantially increased when p53 wild-type (p53(wt)) GBMs were cotreated with the active form of p53 inhibitor, and this heightened cytotoxic response was accompanied by increased poly(ADP-ribose) polymerase cleavage as well as elevated cellular phospho-H2AX. Analysis of the same series of GBMs, as intracranial xenografts in athymic mice, and administering corresponding p53 inhibitor precursor, which is converted to the active compound in vivo, yielded results consistent with the in vitro analyses: TMZ + p53 inhibitor precursor cotreatment of three distinct p53(wt) GBM xenografts resulted in significant enhancement of TMZ antitumor effect relative to treatment with TMZ alone, as indicated by serial bioluminescence monitoring as well as survival analysis (P < 0.001 for cotreatment survival benefit in each case). Mice receiving intracranial injection with p53(null) GBM showed similar survival benefit from TMZ treatment regardless of the presence or absence of p53 inhibitor precursor. In total, our results indicate that the p53 active and precursor inhibitor pair enhances TMZ cytotoxicity in vitro and in vivo, respectively, and do so in a p53-dependent manner.
Figures


References
-
- Roth JA, Nguyen D, Lawrence DD, et al. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat Med. 1996;2:985–91. - PubMed
-
- Levesque AA, Eastman A. p53-based cancer therapies: Is defective p53 the Achilles heel of the tumor? Carcinogenesis. 2007;28:13–20. - PubMed
-
- Johnson KR, Fan W. Reduced expression of p53 and p21WAF1/CIP1 sensitizes human breast cancer cells to paclitaxel and its combination with 5-fluorouracil. Anticancer Res. 2002;22:3197–204. - PubMed
-
- Wang Y, Zhu S, Cloughesy TF, Liau LM, Mischel PS. p53 disruption profoundly alters the response of human glioblastoma cells to DNA topoisomerase I inhibition. Oncogene. 2004;23:1283–90. - PubMed
-
- Kuo PC, Liu HF, Chao JI. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J Biol Chem. 2004;279:55875–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

