Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl(-)-dependent TauT (SLC6A6)
- PMID: 19074966
- PMCID: PMC2669967
- DOI: 10.1113/jphysiol.2008.164228
Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl(-)-dependent TauT (SLC6A6)
Abstract
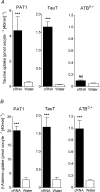
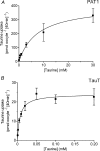
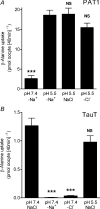
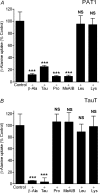
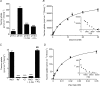
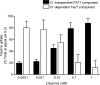
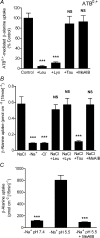
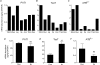
Taurine is an essential amino acid in some mammals and is conditionally essential in humans. Taurine is an abundant component of meat and fish-based foods and has been used as an oral supplement in the treatment of disorders such as cystic fibrosis and hypertension. The purpose of this investigation was to identity the relative contributions of the solute transporters involved in taurine uptake across the luminal membrane of human enterocytes. Distinct transport characteristics were revealed following expression of the candidate solute transporters in Xenopus laevis oocytes: PAT1 (SLC36A1) is a H(+)-coupled, pH-dependent, Na(+)- and Cl(-)-independent, low-affinity, high-capacity transporter for taurine and beta-alanine; TauT (SLC6A6) is a Na(+)- and Cl(-)-dependent, high-affinity, low-capacity transporter of taurine and beta-alanine; ATB(0,+) (SLC6A14) is a Na(+)- and Cl(-)-dependent, high-affinity, low-capacity transporter which accepts beta-alanine but not taurine. Taurine uptake across the brush-border membrane of human intestinal Caco-2 cell monolayers showed characteristics of both PAT1- and TauT-mediated transport. Under physiological conditions, Cl(-)-dependent TauT-mediated uptake predominates at low taurine concentrations, whereas at higher concentrations typical of diet, Cl(-)-independent PAT1-mediated uptake is the major absorptive mechanism. Real-time PCR analysis of human duodenal and ileal biopsy samples demonstrates that PAT1, TauT and ATB(0,+) mRNA are expressed in each tissue but to varying degrees. In conclusion, this study is the first to demonstrate both taurine uptake via PAT1 and functional coexpression of PAT1 and TauT at the apical membrane of the human intestinal epithelium. PAT1 may be responsible for bulk taurine uptake during a meal whereas TauT may be important for taurine supply to the intestinal epithelium and for taurine capture between meals.
Figures
References
-
- Anderson CMH, Ganapathy V, Thwaites DT. High- and low-capacity taurine transport across the luminal membrane of the small intestinal epithelium. FASEB J. 2008a;22:1202.3.
-
- Anderson CMH, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, Munck LK, Miyauchi S, Taylor PM, Campbell FC, Munck BG, Daniel H, Ganapathy V, Thwaites DT. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. - PubMed
-
- Anderson CMH, Thwaites DT. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1) J Cell Physiol. 2005;204:604–613. - PubMed
-
- Balesaria S, Pell RJ, Abbott LJ, Tasleem A, Chavele KM, Barley NF, Khair U, Simon A, Moriarty KJ, Brydon WG, Walters JRF. Exploring possible mechanisms for primary bile acid malabsorption: evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhoea. Eur J Gastroenterol Hepatol. 2008;20:413–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

