The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation
- PMID: 19074970
- PMCID: PMC2670080
- DOI: 10.1113/jphysiol.2008.161737
The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation
Abstract
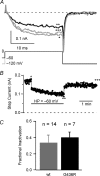
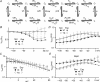
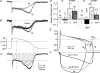
Timothy syndrome (TS) is a multiorgan dysfunction caused by a Gly to Arg substitution at position 406 (G406R) of the human CaV1.2 (L-type) channel. The TS phenotype includes severe arrhythmias that are thought to be triggered by impaired open-state voltage-dependent inactivation (OSvdI). The effect of the TS mutation on other L-channel gating mechanisms has yet to be investigated. We compared kinetic properties of exogenously expressed (HEK293 cells) rabbit cardiac L-channels with (G436R; corresponding to position 406 in human clone) and without (wild-type) the TS mutation. Our results surprisingly show that the TS mutation did not affect close-state voltage-dependent inactivation, which suggests different gating mechanisms underlie these two types of voltage-dependent inactivation. The TS mutation also significantly slowed activation at voltages less than 10 mV, and significantly slowed deactivation across all test voltages. Deactivation was slowed in the double mutant G436R/S439A, which suggests that phosphorylation of S439 was not involved. The L-channel agonist Bay K8644 increased the magnitude of both step and tail currents, but surprisingly failed to slow deactivation of TS channels. Our mathematical model showed that slowed deactivation plus impaired OSvdI combine to synergistically increase cardiac action potential duration that is a likely cause of arrhythmias in TS patients. Roscovitine, a tri-substituted purine that enhances L-channel OSvdI, restored TS-impaired OSvdI. Thus, inactivation-enhancing drugs are likely to improve cardiac arrhythmias and other pathologies afflicting TS patients.
Figures

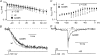


References
-
- Benson C, White J, De Bono J, O’Donnell A, Raynaud F, Cruickshank C, McGrath H, Walton M, Workman P, Kaye S, Cassidy J, Gianella-Borradori A, Judson I, Twelves C. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. - PMC - PubMed
-
- Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002;90:14–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

