Expansion of human regulatory T-cells from patients with type 1 diabetes
- PMID: 19074986
- PMCID: PMC2646064
- DOI: 10.2337/db08-1168
Expansion of human regulatory T-cells from patients with type 1 diabetes
Abstract
Objective: Regulatory T-cells (Tregs) have catalyzed the field of immune regulation. However, translating Treg-based therapies from animal models of autoimmunity to human clinical trials requires robust methods for the isolation and expansion of these cells-a need forming the basis for these studies.
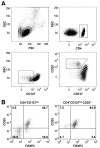
Research design and methods: Tregs from recent-onset type 1 diabetic patients and healthy control subjects were isolated by fluorescence-activated cell sorting and compared for their capacity to expand in vitro in response to anti-CD3-anti-CD28-coated microbeads and IL-2. Expanded cells were examined for suppressive function, lineage markers and FOXP3, and cytokine production.
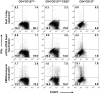
Results: Both CD4+CD127(lo/-) and CD4+CD127(lo/-)CD25+ T-cells could be expanded and used as Tregs. However, expansion of CD4+CD127(lo/-) cells required the addition of rapamycin to maintain lineage purity. In contrast, expansion of CD4+CD127(lo/-)CD25+ T-cells, especially the CD45RA+ subset, resulted in high yield, functional Tregs that maintained higher FOXP3 expression in the absence of rapamycin. Tregs from type 1 diabetic patients and control subjects expanded similarly and were equally capable of suppressing T-cell proliferation. Regulatory cytokines were produced by Tregs after culture; however, a portion of FOXP3+ cells were capable of producing interferon (IFN)-gamma after reactivation. IFN-gamma production was observed from both CD45RO+ and CD45RA+ Treg populations.
Conclusions: The results support the feasibility of isolating Tregs for in vitro expansion. Based on expansion capacity, FOXP3 stability, and functional properties, the CD4+CD127(lo/-)CD25+ T-cells represent a viable cell population for cellular therapy in this autoimmune disease.
Figures




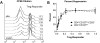

References
-
- Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358: 221–229, 2001 - PubMed
-
- Chatenoud L, Salomon B, Bluestone JA: Suppressor T cells: they're back and critical for regulation of autoimmunity! Immunol Rev 182: 149–163, 2001 - PubMed
-
- Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD: B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD. Ig mu null mice. J Exp Med 184: 2049–2053, 1996 - PMC - PubMed
-
- Fontenot JD, Rudensky AY: Molecular aspects of regulatory T cell development. Semin Immunol 16: 73–80, 2004 - PubMed
-
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA: B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12: 431–440, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

