Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process
- PMID: 19075009
- PMCID: PMC2643801
- DOI: 10.1128/MCB.01227-08
Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process
Abstract
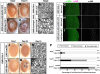
The intracellular accumulation of unfolded or misfolded proteins is believed to contribute to aging and age-related neurodegenerative diseases. However, the links between age-dependent proteotoxicity and cellular protein degradation systems remain poorly understood. Here, we show that 26S proteasome activity and abundance attenuate with age, which is associated with the impaired assembly of the 26S proteasome with the 19S regulatory particle (RP) and the 20S proteasome. In a genetic gain-of-function screen, we characterized Rpn11, which encodes a subunit of the 19S RP, as a suppressor of expanded polyglutamine-induced progressive neurodegeneration. Rpn11 overexpression suppressed the age-related reduction of the 26S proteasome activity, resulting in the extension of flies' life spans with suppression of the age-dependent accumulation of ubiquitinated proteins. On the other hand, the loss of function of Rpn11 caused an early onset of reduced 26S proteasome activity and a premature age-dependent accumulation of ubiquitinated proteins. It also caused a shorter life span and an enhanced neurodegenerative phenotype. Our results suggest that maintaining the 26S proteasome with age could extend the life span and suppress the age-related progression of neurodegenerative diseases.
Figures



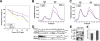

References
-
- Bence, N. F., R. M. Sampat, and R. R. Kopito. 2001. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 2921552-1555. - PubMed
-
- Chondrogianni, N., and E. S. Gonos. 2005. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp. Gerontol. 40931-938. - PubMed
-
- Cummings, C. J., E. Reinstein, Y. Sun, B. Antalffy, Y. Jiang, A. Ciechanover, H. T. Orr, A. L. Beaudet, and H. Y. Zoghbi. 1999. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron 24879-892. - PubMed
-
- Fernandez-Funez, P., M. L. Nino-Rosales, B. de Gouyon, W. C. She, J. M. Luchak, P. Martinez, E. Turiegano, J. Benito, M. Capovilla, P. J. Skinner, A. McCall, I. Canal, H. T. Orr, H. Y. Zoghbi, and J. Botas. 2000. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408101-106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
