Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis
- PMID: 19075027
- PMCID: PMC2643639
- DOI: 10.1128/IAI.01192-08
Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis
Retraction in
-
Retraction for Hunter et al., "Lactobacillus bulgaricus Prevents Intestinal Epithelial Cell Injury Caused by Enterobacter sakazakii-Induced Nitric Oxide both In Vitro and in the Newborn Rat Model of Necrotizing Enterocolitis".Infect Immun. 2018 May 22;86(6):e00212-18. doi: 10.1128/IAI.00212-18. Print 2018 Jun. Infect Immun. 2018. PMID: 29789433 Free PMC article. No abstract available.
Expression of concern in
-
Publisher's Expression of Concern: Lactobacillus bulgaricus Prevents Intestinal Epithelial Cell Injury Caused by Enterobacter sakazakii-Induced Nitric Oxide both In Vitro and in the Newborn Rat Model of Necrotizing Enterocolitis.Infect Immun. 2017 Mar 23;85(4):e00054-17. doi: 10.1128/IAI.00054-17. Print 2017 Apr. Infect Immun. 2017. PMID: 28336717 Free PMC article. No abstract available.
Abstract
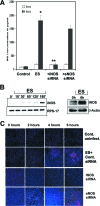
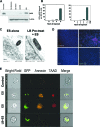
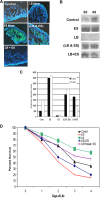
Enterobacter sakazakii is an emerging pathogen that has been associated with outbreaks of necrotizing enterocolitis (NEC) as well as infant sepsis and meningitis. Our previous studies demonstrated that E. sakazakii induces NEC in a newborn rat model by inducing enterocyte apoptosis. However, the mechanisms responsible for enterocyte apoptosis are not known. Here we demonstrate that E. sakazakii induces significant production of nitric oxide (NO) in rat intestinal epithelial cells (IEC-6) upon infection. The elevated production of NO, which is due to increased expression of inducible NO synthase, is responsible for apoptosis of IEC-6 cells. Notably, pretreatment of IEC-6 cells with Lactobacillus bulgaricus (ATCC 12278) attenuated the upregulation of NO production and thereby protected the cells from E. sakazakii-induced apoptosis. Furthermore, pretreatment with L. bulgaricus promoted the integrity of enterocytes both in vitro and in the infant rat model of NEC, even after challenge with E. sakazakii. Infection of IEC-6 cells with E. sakazakii upregulated several genes related to apoptosis, cytokine production, and various signaling pathways, as demonstrated by rat gene array analysis, and this upregulation was subdued by pretreatment with L. bulgaricus. In agreement with these data, L. bulgaricus pretreatment protected newborn rats infected with E. sakazakii from developing NEC, resulting in improved survival.
Figures



References
-
- Barclay, A. R., B. Stenson, J. H. Simpson, L. T. Weaver, and W. C. Wilson. 2007. Probiotics for necrotizing enterocolitis: a systematic review. J. Pediatr. Gastroenterol. Nutr. 45569-576. - PubMed
-
- Bin-Nun, A., R. Bromiker, M. Wilschanski, M. Kaplan, B. Rudensky, M. Caplan, and C. Hammerman. 2005. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J. Pediatr. 147192-196. - PubMed
-
- Dani, C., R. Biadaioli, G. Bertini, E. Martelli, and F. F. Rubaltelli. 2002. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol. Neonate 82103-108. - PubMed
-
- Doran, S., and S. L. Gorbach. 2006. Probiotics: their role in the treatment and prevention of disease. Expert Rev. Anti Infect. Ther. 4261-275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

