Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers
- PMID: 19075045
- PMCID: PMC2650585
- DOI: 10.1128/AAC.01034-08
Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers
Abstract
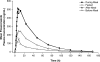
A four-part, randomized, crossover study with healthy subjects evaluated the effects of gastric pH, the dosing frequency and prandial state, food consumption timing, and gastric motility on the absorption of posaconazole. In part 1, a single dose (SD) of posaconazole (400 mg) was administered alone or with an acidic beverage or a proton pump inhibitor (PPI), or both. In part 2, posaconazole (400 mg twice daily and 200 mg four times daily) was administered for 7 days with and without a nutritional supplement (Boost). In part 3, an SD of posaconazole (400 mg) was administered while the subjects were fasting and before, during, and after a high-fat meal. In part 4, an SD of posaconazole (400 mg) and the nutritional supplement were administered alone, with metoclopramide, and with loperamide. Compared to the results obtained with posaconazole alone, administration with an acidic beverage increased the posaconazole maximum concentration in plasma (C(max)) and the area under the concentration-time curve (AUC) by 92% and 70%, respectively, whereas a higher gastric pH decreased the posaconazole C(max) and AUC by 46% and 32%, respectively. Compared to the results obtained with posaconazole alone, posaconazole at 400 mg or at 200 mg plus the nutritional supplement increased the posaconazole C(max) and AUC by 65% and 66%, respectively, and by up to 137% and 161%, respectively. Administration before a high-fat meal increased the C(max) and the AUC by 96% and 111%, respectively, while administration during and after the meal increased the C(max) and the AUC by up to 339% and 387%, respectively. Increased gastric motility decreased the C(max) and the AUC by 21% and 19%, respectively. Strategies to maximize posaconazole exposure in patients with absorption difficulties include administration with or after a high-fat meal, with any meal or nutritional supplement, with an acidic beverage, or in divided doses and the avoidance of proton pump inhibitors.
Figures




Comment in
-
What should be the first-choice strategy to maximize posaconazole exposure in daily clinical practice?Antimicrob Agents Chemother. 2009 Aug;53(8):3608-9; author reply 609-10. doi: 10.1128/AAC.00256-09. Antimicrob Agents Chemother. 2009. PMID: 19617468 Free PMC article. No abstract available.
References
-
- AstraZeneca. 2008. Prescribing information for Nexium (esomeprazole magnesium) delayed-release capsules/for delayed-release oral suspension. AstraZeneca, Wilmington, DE.
-
- Barone, J. A., J. G. Koh, R. H. Bierman, J. L. Colaizzi, K. A. Swanson, M. C. Gaffar, B. L. Moskovitz, W. Mechlinski, and V. Van de Velde. 1993. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob. Agents Chemother. 37:778-784. - PMC - PubMed
-
- Barone, J. A., B. L. Moskovitz, J. Guarnieri, A. E. Hassell, J. L. Colaizzi, R. H. Bierman, and L. Jessen. 1998. Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers. Pharmacotherapy 18:295-301. - PubMed
-
- Cornely, O. A., J. Maertens, D. J. Winston, J. Perfect, A. J. Ullmann, T. J. Walsh, D. Helfgott, J. Holowiecki, D. Stockelberg, Y.-T. Goh, M. Petrini, C. Hardalo, R. Suresh, and D. Angulo-Gonzalez. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348-359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

