Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection
- PMID: 19075065
- PMCID: PMC2650529
- DOI: 10.1128/AAC.00775-08
Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection
Abstract
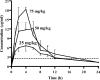
We investigated the activity of linezolid, alone and in combination with rifampin (rifampicin), against a methicillin-resistant Staphylococcus aureus (MRSA) strain in vitro and in a guinea pig model of foreign-body infection. The MIC, minimal bactericidal concentration (MBC) in logarithmic phase, and MBC in stationary growth phase were 2.5, >20, and >20 microg/ml, respectively, for linezolid; 0.01, 0.08, and 2.5 microg/ml, respectively, for rifampin; and 0.16, 0.63, >20 microg/ml, respectively, for levofloxacin. In time-kill studies, bacterial regrowth and the development of rifampin resistance were observed after 24 h with rifampin alone at 1x or 4x the MIC and were prevented by the addition of linezolid. After the administration of single intraperitoneal doses of 25, 50, and 75 mg/kg of body weight, linezolid peak concentrations of 6.8, 12.7, and 18.1 microg/ml, respectively, were achieved in sterile cage fluid at approximately 3 h. The linezolid concentration remained above the MIC of the test organism for 12 h with all doses. Antimicrobial treatments of animals with cage implant infections were given twice daily for 4 days. Linezolid alone at 25, 50, and 75 mg/kg reduced the planktonic bacteria in cage fluid during treatment by 1.2 to 1.7 log(10) CFU/ml; only linezolid at 75 mg/kg prevented bacterial regrowth 5 days after the end of treatment. Linezolid used in combination with rifampin (12.5 mg/kg) was more effective than linezolid used as monotherapy, reducing the planktonic bacteria by >or=3 log(10) CFU (P < 0.05). Efficacy in the eradication of cage-associated infection was achieved only when linezolid was combined with rifampin, with cure rates being between 50% and 60%, whereas the levofloxacin-rifampin combination demonstrated the highest cure rate (91%) against the strain tested. The linezolid-rifampin combination is a treatment option for implant-associated infections caused by quinolone-resistant MRSA.
Figures



References
-
- Acocella, G. 1983. Pharmacokinetics and metabolism of rifampin in humans. Rev. Infect. Dis. 5(Suppl. 3):S428-S432. - PubMed
-
- Bassetti, M., A. Di Biagio, G. Cenderello, V. Del Bono, A. Palermo, M. Cruciani, and D. Bassetti. 2001. Linezolid treatment of prosthetic hip infections due to methicillin-resistant Staphylococcus aureus (MRSA). J. Infect. 43:148-149. - PubMed
-
- Bonomo, R. A. 2000. Multiple antibiotic-resistant bacteria in long-term-care facilities: an emerging problem in the practice of infectious diseases. Clin. Infect. Dis. 31:1414-1422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

