Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed
- PMID: 19075110
- PMCID: PMC2600750
- DOI: 10.1083/jcb.200810060
Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed
Abstract
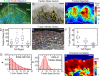
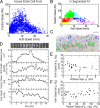
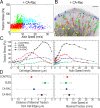
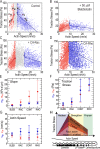
How focal adhesions (FAs) convert retrograde filamentous actin (F-actin) flow into traction stress on the extracellular matrix to drive cell migration is unknown. Using combined traction force and fluorescent speckle microscopy, we observed a robust biphasic relationship between F-actin speed and traction force. F-actin speed is inversely related to traction stress near the cell edge where FAs are formed and F-actin motion is rapid. In contrast, larger FAs where the F-actin speed is low are marked by a direct relationship between F-actin speed and traction stress. We found that the biphasic switch is determined by a threshold F-actin speed of 8-10 nm/s, independent of changes in FA protein density, age, stress magnitude, assembly/disassembly status, or subcellular position induced by pleiotropic perturbations to Rho family guanosine triphosphatase signaling and myosin II activity. Thus, F-actin speed is a fundamental regulator of traction force at FAs during cell migration.
Figures

References
-
- Balaban, N.Q., U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. - PubMed
-
- Bershadsky, A.D., N.Q. Balaban, and B. Geiger. 2003. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19:677–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

