Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3
- PMID: 19075114
- PMCID: PMC2600738
- DOI: 10.1083/jcb.200804003
Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3
Abstract
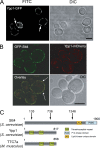
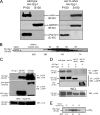
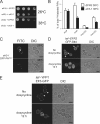
The phosphoinositide phosphatidylinositol 4-phosphate (PtdIns4P) is an essential signaling lipid that regulates secretion and polarization of the actin cytoskeleton. In Saccharomyces cerevisiae, the PtdIns 4-kinase Stt4 catalyzes the synthesis of PtdIns4P at the plasma membrane (PM). In this paper, we identify and characterize two novel regulatory components of the Stt4 kinase complex, Ypp1 and Efr3. The essential gene YPP1 encodes a conserved protein that colocalizes with Stt4 at cortical punctate structures and regulates the stability of this lipid kinase. Accordingly, Ypp1 interacts with distinct regions on Stt4 that are necessary for the assembly and recruitment of multiple copies of the kinase into phosphoinositide kinase (PIK) patches. We identify the membrane protein Efr3 as an additional component of Stt4 PIK patches. Efr3 is essential for assembly of both Ypp1 and Stt4 at PIK patches. We conclude that Ypp1 and Efr3 are required for the formation and architecture of Stt4 PIK patches and ultimately PM-based PtdIns4P signaling.
Figures






References
-
- Aronov, S., and J.E. Gerst. 2004. Involvement of the late secretory pathway in actin regulation and mRNA transport in yeast. J. Biol. Chem. 279:36962–36971. - PubMed
-
- Audhya, A., and S.D. Emr. 2002. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2:593–605. - PubMed
-
- Blatch, G.L., and M. Lassle. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 21:932–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

