Etifoxine improves peripheral nerve regeneration and functional recovery
- PMID: 19075249
- PMCID: PMC2629330
- DOI: 10.1073/pnas.0811201106
Etifoxine improves peripheral nerve regeneration and functional recovery
Abstract
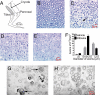
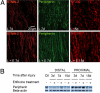
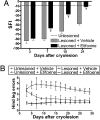
Peripheral nerves show spontaneous regenerative responses, but recovery after injury or peripheral neuropathies (toxic, diabetic, or chronic inflammatory demyelinating polyneuropathy syndromes) is slow and often incomplete, and at present no efficient treatment is available. Using well-defined peripheral nerve lesion paradigms, we assessed the therapeutic usefulness of etifoxine, recently identified as a ligand of the translocator protein (18 kDa) (TSPO), to promote axonal regeneration, modulate inflammatory responses, and improve functional recovery. We found by histologic analysis that etifoxine therapy promoted the regeneration of axons in and downstream of the lesion after freeze injury and increased axonal growth into a silicone guide tube by a factor of 2 after nerve transection. Etifoxine also stimulated neurite outgrowth in PC12 cells, and the effect was even stronger than for specific TSPO ligands. Etifoxine treatment caused a marked reduction in the number of macrophages after cryolesion within the nerve stumps, which was rapid in the proximal and delayed in the distal nerve stumps. Functional tests revealed accelerated and improved recovery of locomotion, motor coordination, and sensory functions in response to etifoxine. This work demonstrates that etifoxine, a clinically approved drug already used for the treatment of anxiety disorders, is remarkably efficient in promoting acceleration of peripheral nerve regeneration and functional recovery. Its possible mechanism of action is discussed, with reference to the neurosteroid concept. This molecule, which easily enters nerve tissues and regulates multiple functions in a concerted manner, offers promise for the treatment of peripheral nerve injuries and axonal neuropathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Barakat-Walter I. Role of thyroid hormones and their receptors in peripheral nerve regeneration. J Neurobiol. 1999;40:541–559. - PubMed
-
- Schumacher M, et al. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res Rev. 2001;37:343–359. - PubMed
-
- Gold BG, Udina E, Bourdette D, Navarro X. Neuroregenerative and neuroprotective actions of neuroimmunophilin compounds in traumatic and inflammatory neuropathies. Neurol Res. 2004;26:371–380. - PubMed
-
- Melcangi RC, Garcia-Segura LM. Therapeutic approaches to peripheral neuropathy based on neuroactive steroids. Expert Rev Neurother. 2006;6:1121–1125. - PubMed
-
- Papadopoulos V, et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

