Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma
- PMID: 19075262
- PMCID: PMC2645859
- DOI: 10.1200/JCO.2008.18.9639
Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma
Abstract
Purpose: This open-label, prospective, single-arm, phase II study combined erlotinib with radiation therapy (XRT) and temozolomide to treat glioblastoma multiforme (GBM) and gliosarcoma. The objectives were to determine efficacy of this treatment as measured by survival and to explore the relationship between molecular markers and treatment response.
Patients and methods: Sixty-five eligible adults with newly diagnosed GBM or gliosarcoma were enrolled. We intended to treat patients not currently treated with enzyme-inducing antiepileptic drugs (EIAEDs) with 100 mg/d of erlotinib during XRT and 150 mg/d after XRT. Patients receiving EIAEDs were to receive 200 mg/d of erlotinib during XRT and 300 mg/d after XRT. After XRT, the erlotinib dose was escalated until patients developed tolerable grade 2 rash or until the maximum allowed dose was reached. All patients received temozolomide during and after XRT. Molecular markers of epidermal growth factor receptor (EGFR), EGFRvIII, phosphatase and tensin homolog (PTEN), and methylation status of the promotor region of the MGMT gene were analyzed from tumor tissue. Survival was compared with outcomes from two historical phase II trials.
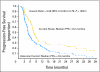
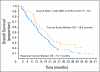
Results: Median survival was 19.3 months in the current study and 14.1 months in the combined historical control studies, with a hazard ratio for survival (treated/control) of 0.64 (95% CI, 0.45 to 0.91). Treatment was well tolerated. There was a strong positive correlation between MGMT promotor methylation and survival, as well as an association between MGMT promotor-methylated tumors and PTEN positivity shown by immunohistochemistry with improved survival.
Conclusion: Patients treated with the combination of erlotinib and temozolomide during and following radiotherapy had better survival than historical controls. Additional studies are warranted.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Chicago, IL: Central Brain Tumor Registry of the United States; 2004. Central Brain Tumor Registry of the United States Statistical Report: Primary Brain Tumors in the United States, 1997-2001.
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. - PubMed
-
- Feldkamp MM, Lala P, Lau N, et al. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

