Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC
- PMID: 19075287
- PMCID: PMC2605222
- DOI: 10.1084/jem.20080718
Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC
Abstract
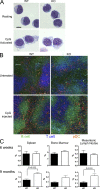
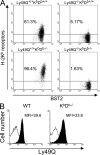
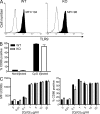
Plasmacytoid dendritic cells (pDCs) are an important source of type I interferon (IFN) during initial immune responses to viral infections. In mice, pDCs are uniquely characterized by high-level expression of Ly49Q, a C-type lectin-like receptor specific for class I major histocompatibility complex (MHC) molecules. Despite having a cytoplasmic immunoreceptor tyrosine-based inhibitory motif, Ly49Q was found to enhance pDC function in vitro, as pDC cytokine production in response to the Toll-like receptor (TLR) 9 agonist CpG-oligonucleotide (ODN) could be blocked using soluble monoclonal antibody (mAb) to Ly49Q or H-2K(b). Conversely, CpG-ODN-dependent IFN-alpha production by pDCs was greatly augmented upon receptor cross-linking using immobilized anti-Ly49Q mAb or recombinant H-2K(b) ligand. Accordingly, Ly49Q-deficient pDCs displayed a severely reduced capacity to produce cytokines in response to TLR7 and TLR9 stimulation both in vitro and in vivo. Finally, TLR9-dependent antiviral responses were compromised in Ly49Q-null mice infected with mouse cytomegalovirus. Thus, class I MHC recognition by Ly49Q on pDCs is necessary for optimal activation of innate immune responses in vivo.
Figures
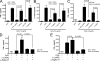



References
-
- Liu, Y.J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275–306. - PubMed
-
- Abb, J., H. Abb, and F. Deinhardt. 1984. Relationship between natural killer (NK) cells and interferon (IFN) alpha-producing cells in human peripheral blood. Studies with a monoclonal antibody with specificity for human natural killer cells. Immunobiology. 167:359–364. - PubMed
-
- Perussia, B., V. Fanning, and G. Trinchieri. 1985. A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat. Immun. Cell Growth Regul. 4:120–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

