Estrogen-dependent enhancement of NO production in the nucleus tractus solitarius contributes to ethanol-induced hypotension in conscious female rats
- PMID: 19076118
- PMCID: PMC2667130
- DOI: 10.1111/j.1530-0277.2008.00845.x
Estrogen-dependent enhancement of NO production in the nucleus tractus solitarius contributes to ethanol-induced hypotension in conscious female rats
Abstract
Background: Our previous pharmacological and cellular studies showed that peripheral (cardiac and vascular) nitric oxide synthase (NOS)-derived NO is implicated in the estrogen (E(2))-dependent hypotensive action of ethanol in female rats. The objective of this study was to test the hypothesis that enhanced NO production in the nucleus tractus solitarius (NTS) is implicated in the E(2)-dependent hypotensive action of ethanol.
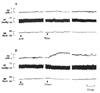
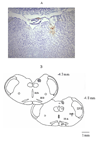
Methods: To achieve this goal, we utilized in vivo electrochemistry to measure real time changes in neuronal NO to investigate the acute effects of intragastric ethanol (0, 0.5, or 1 g/kg) on NO in NTS neurons, blood pressure (BP), and heart rate (HR) in conscious female rats in the absence (ovariectomized, OVX, rats) or presence of E(2).
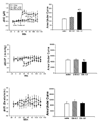
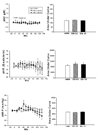
Results: In sham operated (SO) rats, ethanol elicited dose-related increase in NTS NO and reduction in BP. These neurochemical and BP effects of ethanol were absent in OVX rats. Whether the neurochemical effect of ethanol and the associated hypotension are dependent on rapid E(2) signaling was investigated. In OVX rats pretreated, 30 minutes earlier, with E(2) (1 microg/kg), intragastric ethanol (1 g/kg) increased NTS NO and reduced BP and these responses were comparable to those obtained in SO rats.
Conclusions: The present findings suggest that increased production of NO in NTS neurons contributes to ethanol-evoked hypotension in female rats. Further, ethanol enhancement of neuronal NO production in the brainstem is dependent on rapid E(2) signaling.
Figures

Similar articles
-
An association between the estrogen-dependent hypotensive effect of ethanol and an elevated brainstem c-jun mRNA in female rats.Brain Res. 2001 Aug 31;912(1):79-88. doi: 10.1016/s0006-8993(01)02727-5. Brain Res. 2001. PMID: 11520495
-
Estrogen-dependent hypotensive effects of ethanol in conscious female rats.Alcohol Clin Exp Res. 1999 Apr;23(4):624-32. Alcohol Clin Exp Res. 1999. PMID: 10235298
-
Estrogen Receptors α and β Play Major Roles in Ethanol-Evoked Myocardial Oxidative Stress and Dysfunction in Conscious Ovariectomized Rats.Alcohol Clin Exp Res. 2017 Feb;41(2):279-290. doi: 10.1111/acer.13290. Epub 2016 Dec 29. Alcohol Clin Exp Res. 2017. PMID: 28032633 Free PMC article.
-
Brainstem norepinephrine neurons mediate ethanol-evoked pressor response but not baroreflex dysfunction.Alcohol Clin Exp Res. 2005 Apr;29(4):639-47. doi: 10.1097/01.alc.0000160083.72579.ec. Alcohol Clin Exp Res. 2005. PMID: 15834230
-
Brain stem adenosine receptors modulate centrally mediated hypotensive responses in conscious rats: A review.J Adv Res. 2015 May;6(3):331-40. doi: 10.1016/j.jare.2014.12.005. Epub 2014 Dec 18. J Adv Res. 2015. PMID: 26257930 Free PMC article. Review.
Cited by
-
Enhanced hemeoxygenase activity in the rostral ventrolateral medulla mediates exaggerated hemin-evoked hypotension in the spontaneously hypertensive rat.J Pharmacol Exp Ther. 2011 Oct;339(1):267-74. doi: 10.1124/jpet.111.183368. Epub 2011 Jul 18. J Pharmacol Exp Ther. 2011. PMID: 21768222 Free PMC article.
-
Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats.J Pharmacol Exp Ther. 2012 Jun;341(3):579-86. doi: 10.1124/jpet.112.192369. Epub 2012 Feb 24. J Pharmacol Exp Ther. 2012. PMID: 22366659 Free PMC article.
-
The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrena axis responses to intracerebroventricular ethanol administration in the rat.J Neuroendocrinol. 2010 Jan;22(1):24-32. doi: 10.1111/j.1365-2826.2009.01934.x. Epub 2009 Nov 14. J Neuroendocrinol. 2010. PMID: 19912475 Free PMC article.
-
Ovariectomy Exacerbates Acute Ethanol-Induced Tachycardia: Role of Nitric Oxide and NMDA Receptors in the Rostral Ventrolateral Medulla.Int J Mol Sci. 2023 Mar 7;24(6):5087. doi: 10.3390/ijms24065087. Int J Mol Sci. 2023. PMID: 36982161 Free PMC article.
References
-
- Abdel-Rahman AR, Merrill RH, Wooles WR. Effect of acute ethanol administration on the baroreceptor reflex control of heart rate in normotensive human volunteers. Clin Sci (Lond) 1987a;72:113–122. - PubMed
-
- Abdel-Rahman AR, Russ R, Strickland JA, Wooles WR. Acute effects of ethanol on baroreceptor reflex control of heart rate and on pressor and depressor responsiveness in rats. Canadian journal of physiology and pharmacology. 1987b;65:834–841. - PubMed
-
- Al-Rejaie S, Dar MS. Antagonism of ethanol ataxia by intracerebellar nicotine: possible modulation by mouse cerebellar nitric oxide and cGMP. Brain research bulletin. 2006;69:187–196. - PubMed
-
- Davda RK, Chandler LJ, Crews FT, Guzman NJ. Ethanol enhances the endothelial nitric oxide synthase response to agonists. Hypertension. 1993;21:939–943. - PubMed
-
- Dias AC, Vitela M, Colombari E, Mifflin SW. Nitric oxide modulation of glutamatergic, baroreflex, and cardiopulmonary transmission in the nucleus of the solitary tract. American journal of physiology. 2005;288:H256–H262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

