Polymorphisms of drug-metabolizing enzymes (GST, CYP2B6 and CYP3A) affect the pharmacokinetics of thiotepa and tepa
- PMID: 19076156
- PMCID: PMC2668084
- DOI: 10.1111/j.1365-2125.2008.03321.x
Polymorphisms of drug-metabolizing enzymes (GST, CYP2B6 and CYP3A) affect the pharmacokinetics of thiotepa and tepa
Abstract
Aims: Thiotepa is widely used in high-dose chemotherapy. Previous studies have shown relations between exposure and severe organ toxicity. Thiotepa is metabolized by cytochrome P450 and glutathione S-transferase enzymes. Polymorphisms of these enzymes may affect elimination of thiotepa and tepa, its main metabolite. The purpose of this study was to evaluate effects of known allelic variants in CYP2B6, CYP3A4, CYP3A5, GSTA1 and GSTP1 genes on pharmacokinetics of thiotepa and tepa.
Methods: White patients (n = 124) received a high-dose regimen consisting of cyclophosphamide, thiotepa and carboplatin as intravenous infusions. Genomic DNA was analysed using polymerase chain reaction and sequencing. Plasma concentrations of thiotepa and tepa were determined using validated GC and LC-MS/MS methods. Relations between allelic variants and elimination pharmacokinetic parameters were evaluated using nonlinear mixed effects modelling (nonmem).
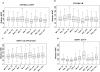
Results: The polymorphisms CYP2B6 C1459T, CYP3A4*1B, CYP3A5*3, GSTA1 (C-69T, G-52A) and GSTP1 C341T had a significant effect on clearance of thiotepa or tepa. Although significant, most effects were generally not large. Clearance of thiotepa and tepa was predominantly affected by GSTP1 C341T polymorphism, which had a frequency of 9.3%. This polymorphism increased non-inducible thiotepa clearance by 52% [95% confidence interval (CI) 41, 64, P < 0.001] and decreased tepa clearance by 32% (95% CI 29, 35, P < 0.001) in heterozygous patients, which resulted in an increase in combined exposure to thiotepa and tepa of 45% in homozygous patients.
Conclusions: This study indicates that the presently evaluated variant alleles explain only a small part of the substantial interindividual variability in thiotepa and tepa pharmacokinetics. Patients homozygous for the GSTP1 C341T allele may have enhanced exposure to thiotepa and tepa.
Figures

Similar articles
-
Influence of polymorphisms of drug metabolizing enzymes (CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, GSTA1, GSTP1, ALDH1A1 and ALDH3A1) on the pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide.Pharmacogenet Genomics. 2008 Jun;18(6):515-23. doi: 10.1097/FPC.0b013e3282fc9766. Pharmacogenet Genomics. 2008. PMID: 18496131
-
Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of thiotepa to TEPA.Cancer Chemother Pharmacol. 2002 Jun;49(6):461-7. doi: 10.1007/s00280-002-0453-3. Epub 2002 Apr 23. Cancer Chemother Pharmacol. 2002. PMID: 12107550
-
Transformation of alkylating regimen of thiotepa into tepa determines the disease progression through GSTP1 gene polymorphism for metastatic breast cancer patients receiving thiotepa containing salvage chemotherapy.Int J Clin Pharmacol Ther. 2015 Nov;53(11):914-22. doi: 10.5414/CP202391. Int J Clin Pharmacol Ther. 2015. PMID: 26396136
-
Substrate specificity, regulation, and polymorphism of human cytochrome P450 2B6.Curr Drug Metab. 2009 Sep;10(7):730-53. doi: 10.2174/138920009789895534. Curr Drug Metab. 2009. PMID: 19702527 Review.
-
Clinical pharmacogenetics and potential application in personalized medicine.Curr Drug Metab. 2008 Oct;9(8):738-84. doi: 10.2174/138920008786049302. Curr Drug Metab. 2008. PMID: 18855611 Review.
Cited by
-
Influence of pharmacogenetics on response and toxicity in breast cancer patients treated with doxorubicin and cyclophosphamide.Br J Cancer. 2010 Mar 16;102(6):1003-9. doi: 10.1038/sj.bjc.6605587. Epub 2010 Feb 23. Br J Cancer. 2010. PMID: 20179710 Free PMC article.
-
Haplotype Inference Using Long-Read Nanopore Sequencing: Application to GSTA1 Promoter.Mol Biotechnol. 2025 Jun;67(6):2512-2519. doi: 10.1007/s12033-024-01213-7. Epub 2024 Jun 17. Mol Biotechnol. 2025. PMID: 38886308 Free PMC article.
-
The analysis of GSTA1 promoter genetic and functional diversity of human populations.Sci Rep. 2021 Mar 3;11(1):5038. doi: 10.1038/s41598-021-83996-2. Sci Rep. 2021. PMID: 33658540 Free PMC article.
-
Genotypes Affecting the Pharmacokinetics of Anticancer Drugs.Clin Pharmacokinet. 2017 Apr;56(4):317-337. doi: 10.1007/s40262-016-0450-z. Clin Pharmacokinet. 2017. PMID: 27641154 Free PMC article. Review.
-
PharmGKB summary: very important pharmacogene information for CYP3A5.Pharmacogenet Genomics. 2012 Jul;22(7):555-8. doi: 10.1097/FPC.0b013e328351d47f. Pharmacogenet Genomics. 2012. PMID: 22407409 Free PMC article. No abstract available.
References
-
- Evans WE, McLeod HL. Pharmacogenomics – drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–49. - PubMed
-
- Rodenhuis S, Baars JW, Schornagel JH, Vlasveld LT, Mandjes I, Pinedo HM. Feasibility and toxicity study of a high-dose chemotherapy regimen for autotransplantation incorporating carboplatin, cyclophosphamide and thiotepa. Ann Oncol. 1992;3:855–60. - PubMed
-
- Miller B, Tenenholz T, Egorin MJ, Sosnovsky G, Rao NU, Gutierrez PL. Cellular pharmacology of N,N′,N″-triethylene thiophosphoramide. Cancer Lett. 1988;41:157–68. - PubMed
-
- Przepiorka D, Madden T, Ippoliti C, Estrov Z, Dimopoulos M. Dosing of thioTEPA for myeloablative therapy. Cancer Chemother Pharmacol. 1995;37:155–60. - PubMed
-
- Huitema ADR, Smits KD, Mathot RA, Schellens JH, Rodenhuis S, Beijnen JH. The clinical pharmacology of alkylating agents in high-dose chemotherapy. Anticancer Drugs. 2000;11:515–33. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

