Exposure to antibacterial agents with QT liability in 14 European countries: trends over an 8-year period
- PMID: 19076158
- PMCID: PMC2668089
- DOI: 10.1111/j.1365-2125.2008.03319.x
Exposure to antibacterial agents with QT liability in 14 European countries: trends over an 8-year period
Abstract
Aims: (i) To classify antibacterial agents with QT liability on the basis of the available evidence, and (ii) to assess trends in their consumption over an 8-year period (1998-2005) in 14 European countries.
Methods: Current published evidence on QT liability of antibiotics was retrieved through MEDLINE search and joined to official warnings from regulatory agencies. Each drug was classified according to an already proposed algorithm based on the strength of evidence: from group A (any evidence) to group E (clinical reports of torsades de pointes and warnings on QT liability). Consumption data were provided by the European Surveillance of Antibacterial Consumption (ESAC) project and were expressed as defined daily doses per 1000 inhabitants per day (DID).
Results: Among 21 detected compounds, nine [six fluoroquinolones (FQs) and three macrolides (MACs)] belonged to group E. Use of group E drugs ranged from 1.3 (Sweden) to 4.1 DID (Italy) in 1998 and from 1.2 (Sweden) to 6.5 DID (Italy) in 2005. Significant exposure was observed in Italy and Spain (6.5 and 3.8 DID, respectively, in 2005). Only Denmark, Sweden and UK showed a slight decrease in use. Exposure to clarithromycin increased in 10 out of 14 countries, with a marked increment in Italy (3 DID in 2005).
Conclusions: Notwithstanding regulatory measures, in 2005 there was still significant exposure to antibacterials with strong evidence of QT liability and, in most countries, it was even increased. This warrants further investigation of appropriateness of use and suggests closer monitoring of group E drugs. Physicians should be aware when prescribing them to susceptible patients.
Figures
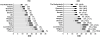
 ); Group E, strongest level of evidence on QT-liability (▪). Percentage: antibacterial agents labeled as group E/total consumption of antibacterials. In parenthesis changes in use of group E compounds (absolute values); + = increment in consumption; − = decrease in consumption
); Group E, strongest level of evidence on QT-liability (▪). Percentage: antibacterial agents labeled as group E/total consumption of antibacterials. In parenthesis changes in use of group E compounds (absolute values); + = increment in consumption; − = decrease in consumption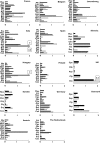
 )
)Similar articles
-
Non-antiarrhythmic drugs prolonging the QT interval: considerable use in seven countries.Br J Clin Pharmacol. 2002 Aug;54(2):171-7. doi: 10.1046/j.1365-2125.2002.01617.x. Br J Clin Pharmacol. 2002. PMID: 12207637 Free PMC article.
-
Hospital consumption of antibiotics in 15 European countries: results of the ESAC Retrospective Data Collection (1997-2002).J Antimicrob Chemother. 2006 Jul;58(1):159-67. doi: 10.1093/jac/dkl147. Epub 2006 May 12. J Antimicrob Chemother. 2006. PMID: 16698845
-
Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe.PLoS One. 2015 Mar 18;10(3):e0119551. doi: 10.1371/journal.pone.0119551. eCollection 2015. PLoS One. 2015. PMID: 25785934 Free PMC article.
-
Arrhythmias associated with fluoroquinolone therapy.Int J Antimicrob Agents. 2007 Apr;29(4):374-9. doi: 10.1016/j.ijantimicag.2006.11.011. Epub 2007 Jan 22. Int J Antimicrob Agents. 2007. PMID: 17241772 Review.
-
Safety of non-antiarrhythmic drugs that prolong the QT interval or induce torsade de pointes: an overview.Drug Saf. 2002;25(4):263-86. doi: 10.2165/00002018-200225040-00004. Drug Saf. 2002. PMID: 11994029 Review.
Cited by
-
QT interval shortening in spontaneous reports submitted to the FDA: the need for consensus.Br J Clin Pharmacol. 2011 Nov;72(5):839-41. doi: 10.1111/j.1365-2125.2011.04065.x. Br J Clin Pharmacol. 2011. PMID: 21777270 Free PMC article. No abstract available.
-
Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe.PLoS One. 2013 Nov 20;8(11):e81208. doi: 10.1371/journal.pone.0081208. eCollection 2013. PLoS One. 2013. PMID: 24278396 Free PMC article.
-
Assessing liver injury associated with antimycotics: Concise literature review and clues from data mining of the FAERS database.World J Hepatol. 2014 Aug 27;6(8):601-12. doi: 10.4254/wjh.v6.i8.601. World J Hepatol. 2014. PMID: 25232453 Free PMC article.
-
Determinants of torsades de pointes in older patients with drug-associated long QT syndrome: a case-control study.Drugs Aging. 2014 Aug;31(8):601-9. doi: 10.1007/s40266-014-0188-y. Drugs Aging. 2014. PMID: 24923384
References
-
- De Ponti F, Poluzzi E, Montanaro NQ. T-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur J Clin Pharmacol. 2000;56:1–18. - PubMed
-
- ICH Topic S 7 B – The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization QT Interval Prolongation) by Human Pharmaceuticals. London: EMEA; 2005. - PubMed
-
- ICH Topic E 14 – The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. London: EMEA; 2005. - PubMed
-
- Dessertenne F [Ventricular tachycardia with 2 variable opposing foci] Arch Mal Coeur Vaiss. 1966;59:263–72. - PubMed
-
- Recanatini M, Poluzzi E, Masetti M, Cavalli A, de Ponti F. QT prolongation through hERG K+ channel blockade: current knowledge and strategies for the early prediction during drug development. Med Res Rev. 2005;25:133–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

