Similar [DE]XXXL[LI] motifs differentially target GLUT8 and GLUT12 in Chinese hamster ovary cells
- PMID: 19076329
- PMCID: PMC2983051
- DOI: 10.1111/j.1600-0854.2008.00866.x
Similar [DE]XXXL[LI] motifs differentially target GLUT8 and GLUT12 in Chinese hamster ovary cells
Abstract
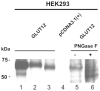
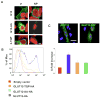
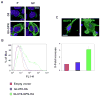
The transport of glucose across cell membranes is mediated by facilitative glucose transporters (GLUTs). The recently identified class III GLUT12 is predominantly expressed in insulin-sensitive tissues such as heart, fat and skeletal muscle. We examined the subcellular localization of GLUT12 in Chinese hamster ovary and human embryonic kidney 293 cells stably expressing murine GLUT12. We have previously shown that another class III GLUT8 contains a [DE]XXXL[LI] motif that directs it to late endosomal/lysosomal compartments. Despite also having this highly conserved motif in its amino terminus, GLUT12 does not colocalize with GLUT8. Rather, GLUT12 resides in the Golgi network and at the plasma membrane (PM). Furthermore, GLUT8 and GLUT12 exhibit dramatic differences in trafficking from the PM. Whereas GLUT8 is internalized following its expression at the cell surface, GLUT12 remains largely associated with the PM. To further explore the trafficking mechanisms, we created mutant constructs to explore the potential role of GLUT12's NH(2)-terminal dileucine motif in regulating its intracellular sorting. We show that both the GPN and the LL residues within the [DE]XXXL[LI] motif influence the cell surface expression of GLUT12 and conclude that the mechanisms governing the intracellular sorting of GLUT12 are distinct from those regulating the sorting of GLUT8.
Figures




References
-
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Molecular membrane biology. 2001;18(4):247–256. - PubMed
-
- Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447(5):480–489. - PubMed
-
- Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab. 2002;282(3):E733–738. - PubMed
-
- Macheda ML, Kelly DJ, Best JD, Rogers S. Expression during rat fetal development of GLUT12--a member of the class III hexose transporter family. Anat Embryol (Berl) 2002;205(5-6):441–452. - PubMed
-
- Zhou Y, Kaye PL, Pantaleon M. Identification of the facilitative glucose transporter 12 gene Glut12 in mouse preimplantation embryos. Gene Expr Patterns. 2004;4(6):621–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

