The role of mitochondria in reactive oxygen species metabolism and signaling
- PMID: 19076429
- PMCID: PMC2869479
- DOI: 10.1196/annals.1427.015
The role of mitochondria in reactive oxygen species metabolism and signaling
Abstract
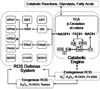
Oxidative stress is considered a major contributor to the etiology of both "normal" senescence and severe pathologies with serious public health implications. Several cellular sources, including mitochondria, are known to produce significant amounts of reactive oxygen species (ROS) that may contribute to intracellular oxidative stress. Mitochondria possess at least 10 known sites that are capable of generating ROS, but they also feature a sophisticated multilayered ROS defense system that is much less studied. This review summarizes the current knowledge about major components involved in mitochondrial ROS metabolism and factors that regulate ROS generation and removal at the level of mitochondria. An integrative systemic approach is applied to analysis of mitochondrial ROS metabolism, which is "dissected" into ROS generation, ROS emission, and ROS scavenging. The in vitro ROS-producing capacity of several mitochondrial sites is compared in the metabolic context and the role of mitochondria in ROS-dependent intracellular signaling is discussed.
Conflict of interest statement
The author declares no conflicts of interest.
Figures




References
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug. Metab. Rev. 2007;39:443–455. - PubMed
-
- Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: a review. Mutagenesis. 2006;21:361–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

