In vitro induction of T cells that are resistant to A2 adenosine receptor-mediated immunosuppression
- PMID: 19076726
- PMCID: PMC2697837
- DOI: 10.1111/j.1476-5381.2008.00019.x
In vitro induction of T cells that are resistant to A2 adenosine receptor-mediated immunosuppression
Abstract
Background and purpose: The increased levels of extracellular adenosine in inflamed tissues down-regulate activated immune cells via the A(2A) adenosine receptor. This A(2A) adenosine receptor-mediated immunosuppression is a disqualifying obstacle in cancer immunotherapy as it protects cancerous tissues from adoptively transferred anti-tumour T cells. The aim of this study was to test whether the negative selection of T cells will produce T cells that are resistant to inhibition by extracellular adenosine.
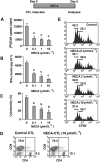
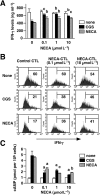
Experimental approach: Cytotoxic T lymphocytes (CTL) were developed by mixed lymphocyte culture in the presence or absence of the adenosine receptor agonist 5'-N-ethylcarboxamidoadenosine (NECA). The sensitivity of CTL to adenosine analogues was characterized by cAMP induction, interferon-gamma production and cytotoxicity.
Key results: CTL that could proliferate even in the presence of NECA were less susceptible to inhibition by A(2A) adenosine receptor agonists, as shown by a much smaller accumulation of cAMP and less inhibition of interferon-gamma production compared with control CTL. The successful protocol to produce CTL that are both resistant to adenosine-mediated immunosuppression and maintain strong cytotoxicity and interferon-gamma secretion required NECA to be added only during the expansion stage after the establishment of CTL. In contrast, the priming of resting T cells in the presence of NECA resulted in T cells with impaired effector functions.
Conclusions and implications: Adenosine-resistant effector T cells were successfully obtained by exposure of activated T cells to NECA. These in vitro studies form the basis for future attempts to produce anti-tumour T cells that are more effective in adoptive immunotherapy.
Figures


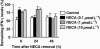
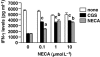
Similar articles
-
A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments.J Immunol. 2009 Nov 1;183(9):5487-93. doi: 10.4049/jimmunol.0901247. J Immunol. 2009. PMID: 19843934
-
Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts.J Mol Cell Cardiol. 2009 Nov;47(5):684-90. doi: 10.1016/j.yjmcc.2009.08.009. Epub 2009 Aug 18. J Mol Cell Cardiol. 2009. PMID: 19695259 Free PMC article.
-
A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells.J Immunol. 2005 Jan 15;174(2):1073-80. doi: 10.4049/jimmunol.174.2.1073. J Immunol. 2005. PMID: 15634932
-
Regulation of lymphocyte function by adenosine.Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2097-103. doi: 10.1161/ATVBAHA.111.226837. Epub 2012 Jul 5. Arterioscler Thromb Vasc Biol. 2012. PMID: 22772752 Free PMC article. Review.
-
The adenosinergic immunomodulatory drugs.Curr Opin Pharmacol. 2009 Aug;9(4):501-6. doi: 10.1016/j.coph.2009.05.005. Epub 2009 Jun 17. Curr Opin Pharmacol. 2009. PMID: 19539527 Free PMC article. Review.
Cited by
-
Purinergic regulation of the immune system.Nat Rev Immunol. 2016 Mar;16(3):177-92. doi: 10.1038/nri.2016.4. Nat Rev Immunol. 2016. PMID: 26922909 Review.
-
Beyond the antigen receptor: editing the genome of T-cells for cancer adoptive cellular therapies.Front Immunol. 2013 Aug 5;4:221. doi: 10.3389/fimmu.2013.00221. eCollection 2013. Front Immunol. 2013. PMID: 23935598 Free PMC article.
-
Hypoxia-induced and A2A adenosine receptor-independent T-cell suppression is short lived and easily reversible.Int Immunol. 2014 Feb;26(2):83-91. doi: 10.1093/intimm/dxt045. Epub 2013 Oct 22. Int Immunol. 2014. PMID: 24150242 Free PMC article.
-
Receptor desensitization and blockade of the suppressive effects of prostaglandin E(2) and adenosine on the cytotoxic activity of human melanoma-infiltrating T lymphocytes.Cancer Immunol Immunother. 2011 Jan;60(1):111-22. doi: 10.1007/s00262-010-0924-z. Epub 2010 Oct 20. Cancer Immunol Immunother. 2011. PMID: 20960188 Free PMC article.
-
Extracellular adenosine controls NKT-cell-dependent hepatitis induction.Eur J Immunol. 2014 Apr;44(4):1119-29. doi: 10.1002/eji.201343866. Epub 2014 Feb 19. Eur J Immunol. 2014. PMID: 24448964 Free PMC article.
References
-
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. - PubMed
-
- von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

