Critical role for constitutive type I interferon signaling in the prevention of cellular transformation
- PMID: 19076978
- PMCID: PMC11158082
- DOI: 10.1111/j.1349-7006.2008.01051.x
Critical role for constitutive type I interferon signaling in the prevention of cellular transformation
Abstract
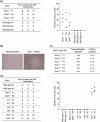
Interferons-alpha/beta, which are produced upon viral infection, are key soluble factors for the establishment of an antiviral state, but are also produced at low levels in the absence of infection. Herein, we demonstrate that a weak signal by these constitutively produced IFN-alpha/beta show a preventive role in cellular transformation. Ifnar1-deficient (Ifnar1(-/-)) MEF, which are devoid of IFN-alpha/beta signal, undergo a spontaneous transformation during long-term cell culture. Similar to Irf1(-/-) MEF, primary Ifnar1(-/-) MEF become tumorigenic in nude mice by the expression of activated c-Ha-Ras oncoprotein. However, Ifnar1(-/-) MEF do not show any abnormal growth properties. A similar observation is made in Ifnb(-/-) MEF that fail to produce constitutive IFN-alpha/beta, whereas such a transforming property is not found in MEF that lack any of the IFN receptor downstream molecules including Stat1, IRF9 and IRF1. Furthermore, Ifnar1(-/-) mice develop chemically-induced skin papilloma more severely than wild-type mice. In addition, the expression levels of IFNAR1 mRNA are significantly decreased in human gastric cancer tissues. These results suggest a cell-intrinsic role of the weak signal by constitutively produced IFN-alpha/beta to prevent cells from transformation, which may be mediated by a hitherto-unknown pathway(s) downstream of the IFN-alpha/beta receptor.
Figures




References
-
- Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell 1994; 77: 391–400. - PubMed
-
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem 1998; 67: 227–64. - PubMed
-
- Uze G, Lutfalla G, Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell 1990; 60: 225–34. - PubMed
-
- Bluyssen AR, Durbin JE, Levy DE. ISGF3 gamma p48, a specificity switch for interferon activated transcription factors. Cytokine Growth Factor Rev 1996; 7: 11–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

