Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain
- PMID: 19077109
- PMCID: PMC2693902
- DOI: 10.1111/j.1365-2990.2008.01006.x
Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain
Abstract
Aims: Microglia are involved in neurodegeneration, are prime targets for anti-inflammatory therapy and are potential biomarkers of disease progression. For example, positron emission tomography imaging employing radioligands for the mitochondrial translocator protein of 18 kDa (TSPO, formerly known as the peripheral benzodiazepine receptor) is being scrutinized to detect neuroinflammation in various diseases. TSPO is presumably present in activated microglia, but may be present in other neural cells.
Methods: We sought to elucidate the protein expression in normal human central nervous system, several neurological diseases (HIV encephalitis, Alzheimer's disease, multiple sclerosis and stroke) and simian immunodeficiency virus encephalitis by performing immunohistochemistry with two anti-TSPO antibodies.
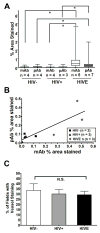
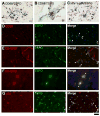
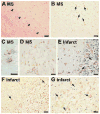
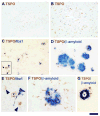
Results: Although the overall parenchymal staining was minimal in normal brain, endothelial and smooth muscle cells, subpial glia, intravascular monocytes and ependymal cells were TSPO-positive. In disease states, elevated TSPO was present in parenchymal microglia, macrophages and some hypertrophic astrocytes, but the distribution of TSPO varied depending on the disease, disease stage and proximity to the lesion or relation to infection. Staining with the two antibodies correlated well in white matter, but one antibody also stained cortical neurones. Quantitative analysis demonstrated a significant increase in TSPO in the white matter of HIV encephalitis compared with brains without encephalitis. TSPO expression was also increased in simian immunodeficiency virus encephalitis.
Conclusions: This report provides the first comprehensive immunohistochemical analysis of the expression of TSPO. The results are useful for informing the usage of positron emission tomography as an imaging modality and have an impact on the potential use of TSPO as an anti-inflammatory pharmacological target.
Figures



References
-
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. - PubMed
-
- Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147:867–883. - PubMed
-
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. - PubMed
-
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. - PubMed
-
- Lee SC, Cosenza MA, Si Q, Rivieccio M, Brosnan CF. The CNS: Cells, Tissues, and Reactions to Insult. In: Ransohoff RM, Benveniste EN, editors. Cytokines and the CNS. 2. Boca Raton, FL: Taylor & Francis Group; 2006. pp. 1–22.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

