Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone
- PMID: 19077183
- PMCID: PMC2637876
- DOI: 10.1186/1471-2202-9-117
Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone
Abstract
Background: The effect of neurotrophic factors in enhancing stroke-induced neurogenesis in the adult subventricular zone (SVZ) is limited by their poor blood-brain barrier (BBB) permeability.Intranasal administration is a noninvasive and valid method for delivery of neuropeptides into the brain, to bypass the BBB. We investigated the effect of treatment with intranasal transforming growth factor-beta1 (TGF-beta1) on neurogenesis in the adult mouse SVZ following focal ischemia. The modified Neurological Severity Scores (NSS) test was used to evaluate neurological function, and infarct volumes were determined from hematoxylin-stained sections. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) labeling was performed at 7 days after middle cerebral artery occlusion (MCAO). Immunohistochemistry was used to detect bromodeoxyuridine (BrdU) and neuron- or glia-specific markers for identifying neurogenesis in the SVZ at 7, 14, 21, 28 days after MCAO.
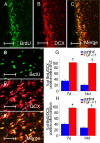
Results: Intranasal treatment of TGF-beta1 shows significant improvement in neurological function and reduction of infarct volume compared with control animals. TGF-beta1 treated mice had significantly less TUNEL-positive cells in the ipsilateral striatum than that in control groups. The number of BrdU-incorporated cells in the SVZ and striatum was significantly increased in the TGF-beta1 treated group compared with control animals at each time point. In addition, numbers of BrdU- labeled cells coexpressed with the migrating neuroblast marker doublecortin (DCX) and the mature neuronal marker neuronal nuclei (NeuN) were significantly increased after intranasal delivery of TGF-beta1, while only a few BrdU labeled cells co-stained with glial fibrillary acidic protein (GFAP).
Conclusion: Intranasal administration of TGF-beta1 reduces infarct volume, improves functional recovery and enhances neurogenesis in mice after stroke. Intranasal TGF-beta1 may have therapeutic potential for cerebrovascular disorders.
Figures





Similar articles
-
Acidic fibroblast growth factor delivered intranasally induces neurogenesis and angiogenesis in rats after ischemic stroke.Neurol Res. 2011 Sep;33(7):675-80. doi: 10.1179/1743132810Y.0000000004. Neurol Res. 2011. PMID: 21756545
-
Blocked angiogenesis in Galectin-3 null mice does not alter cellular and behavioral recovery after middle cerebral artery occlusion stroke.Neurobiol Dis. 2014 Mar;63:155-64. doi: 10.1016/j.nbd.2013.11.003. Epub 2013 Nov 21. Neurobiol Dis. 2014. PMID: 24269916
-
Progranulin promotes functional recovery and neurogenesis in the subventricular zone of adult mice after cerebral ischemia.Brain Res. 2021 Apr 15;1757:147312. doi: 10.1016/j.brainres.2021.147312. Epub 2021 Feb 2. Brain Res. 2021. PMID: 33539798
-
Retinoic acid and environmental enrichment alter subventricular zone and striatal neurogenesis after stroke.Exp Neurol. 2008 Nov;214(1):125-34. doi: 10.1016/j.expneurol.2008.08.006. Epub 2008 Aug 22. Exp Neurol. 2008. PMID: 18778705 Free PMC article.
-
Intranasal application of stem cells and their derivatives as a new hope in the treatment of cerebral hypoxia/ischemia: a review.Rev Neurosci. 2022 Feb 7;33(6):583-606. doi: 10.1515/revneuro-2021-0163. Print 2022 Aug 26. Rev Neurosci. 2022. PMID: 35130375 Review.
Cited by
-
TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke.J Neuroinflammation. 2010 Oct 11;7:62. doi: 10.1186/1742-2094-7-62. J Neuroinflammation. 2010. PMID: 20937129 Free PMC article.
-
Hyperforin improves post-stroke social isolation‑induced exaggeration of PSD and PSA via TGF-β.Int J Mol Med. 2019 Jan;43(1):413-425. doi: 10.3892/ijmm.2018.3971. Epub 2018 Nov 2. Int J Mol Med. 2019. PMID: 30387813 Free PMC article.
-
Microglia as the Critical Regulators of Neuroprotection and Functional Recovery in Cerebral Ischemia.Cell Mol Neurobiol. 2022 Nov;42(8):2505-2525. doi: 10.1007/s10571-021-01145-9. Epub 2021 Aug 30. Cell Mol Neurobiol. 2022. PMID: 34460037 Free PMC article. Review.
-
Stem cell therapy in intracerebral hemorrhage rat model.World J Stem Cells. 2015 Apr 26;7(3):618-29. doi: 10.4252/wjsc.v7.i3.618. World J Stem Cells. 2015. PMID: 25914768 Free PMC article. Review.
-
Parallels between the Developing Vascular and Neural Systems: Signaling Pathways and Future Perspectives for Regenerative Medicine.Adv Sci (Weinh). 2021 Dec;8(23):e2101837. doi: 10.1002/advs.202101837. Epub 2021 Oct 24. Adv Sci (Weinh). 2021. PMID: 34693660 Free PMC article. Review.
References
-
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. - DOI - PMC - PubMed
-
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke; a journal of cerebral circulation. 2005;36:1790–1795. - PubMed
-
- Jin K, Sun Y, Xie L, Childs J, Mao XO, Greenberg DA. Post-ischemic administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF) reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:399–408. doi: 10.1097/00004647-200404000-00005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

