Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm
- PMID: 19077194
- PMCID: PMC2633019
- DOI: 10.1186/1742-4690-5-111
Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm
Abstract
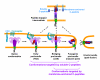
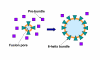
Enveloped viruses encode specialized fusion proteins which promote the merger of viral and cell membranes, permitting the cytosolic release of the viral cores. Understanding the molecular details of this process is essential for antiviral strategies. Recent structural studies revealed a stunning diversity of viral fusion proteins in their native state. In spite of this diversity, the post-fusion structures of these proteins share a common trimeric hairpin motif in which the amino- and carboxy-terminal hydrophobic domains are positioned at the same end of a rod-shaped molecule. The converging hairpin motif, along with biochemical and functional data, implies that disparate viral proteins promote membrane merger via a universal "cast-and-fold" mechanism. According to this model, fusion proteins first anchor themselves to the target membrane through their hydrophobic segments and then fold back, bringing the viral and cellular membranes together and forcing their merger. However, the pathways of protein refolding and the mechanism by which this refolding is coupled to membrane rearrangements are still not understood. The availability of specific inhibitors targeting distinct steps of HIV-1 entry permitted the identification of key conformational states of its envelope glycoprotein en route to fusion. These studies provided functional evidence for the direct engagement of the target membrane by HIV-1 envelope glycoprotein prior to fusion and revealed the role of partially folded pre-hairpin conformations in promoting the pore formation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

