Aurora kinase inhibitors synergize with paclitaxel to induce apoptosis in ovarian cancer cells
- PMID: 19077237
- PMCID: PMC2614415
- DOI: 10.1186/1479-5876-6-79
Aurora kinase inhibitors synergize with paclitaxel to induce apoptosis in ovarian cancer cells
Abstract
Background: A large percentage of patients with recurrent ovarian cancer develop resistance to the taxane class of chemotherapeutics. While mechanisms of resistance are being discovered, novel treatment options and a better understanding of disease resistance are sorely needed. The mitotic kinase Aurora-A directly regulates cellular processes targeted by the taxanes and is overexpressed in several malignancies, including ovarian cancer. Recent data has shown that overexpression of Aurora-A can confer resistance to the taxane paclitaxel.
Methods: We used expression profiling of ovarian tumor samples to determine the most significantly overexpressed genes. In this study we sought to determine if chemical inhibition of the Aurora kinase family using VE-465 could synergize with paclitaxel to induce apoptosis in paclitaxel-resistant and sensitive ovarian cancer cells.
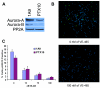
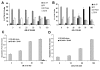
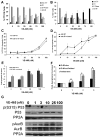
Results: Aurora-A kinase and TPX2, an activator of Aurora-A, are two of the most significantly overexpressed genes in ovarian carcinomas. We show that inhibition of the Aurora kinases prevents phosphorylation of a mitotic marker and demonstrate a dose-dependent increase of apoptosis in treated ovarian cancer cells. We demonstrate at low doses that are specific to Aurora-A, VE-465 synergizes with paclitaxel to induce 4.5-fold greater apoptosis than paclitaxel alone in 1A9 cells. Higher doses are needed to induce apoptosis in paclitaxel-resistant PTX10 cells.
Conclusion: Our results show that VE-465 is a potent killer of taxane resistant ovarian cancer cells and can synergize with paclitaxel at low doses. These data suggest patients whose tumors exhibit high Aurora-A expression may benefit from a combination therapy of taxanes and Aurora-A inhibition.
Figures

References
-
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

