Prolyl isomerase Pin1 is highly expressed in Her2-positive breast cancer and regulates erbB2 protein stability
- PMID: 19077306
- PMCID: PMC2632646
- DOI: 10.1186/1476-4598-7-91
Prolyl isomerase Pin1 is highly expressed in Her2-positive breast cancer and regulates erbB2 protein stability
Abstract
Overexpression of HER-2/Neu occurs in about 25-30% of breast cancer patients and is indicative of poor prognosis. While Her2/Neu overexpression is primarily a result of erbB2 amplification, it has recently been recognized that erbB2 levels are also regulated on the protein level. However, factors that regulate Her2/Neu protein stability are less well understood. The prolyl isomerase Pin1 catalyzes the isomerization of specific pSer/Thr-Pro motifs that have been phosphorylated in response to mitogenic signaling. We have previously reported that Pin1-catalyzed post-phosphorylational modification of signal transduction modulates the oncogenic pathways downstream from c-neu. The goal of this study was to examine the expression of prolyl isomerase Pin1 in human Her2+ breast cancer, and to study if Pin1 affects the expression of Her2/Neu itself.
Methods: Immunohistochemistry for Her2 and Pin1 were performed on two hundred twenty-three human breast cancers, with 59% of the specimen from primary cancers and 41% from metastatic sites. Pin1 inhibition was achieved using siRNA in Her2+ breast cancer cell lines, and its effects were studied using cell viability assays, immunoblotting and immunofluorescence.
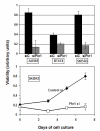
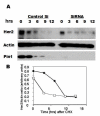
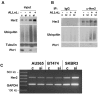
Results: Sixty-four samples (28.7%) stained positive for Her2 (IHC 3+), and 54% (122/223) of all breast cancers stained positive for Pin1. Of the Her2-positive cancers 40 (62.5%) were also Pin1-positive, based on strong nuclear or nuclear and cytoplasmic staining. Inhibition of Pin1 via RNAi resulted in significant suppression of Her2-positive tumor cell growth in BT474, SKBR3 and AU565 cells. Pin1 inhibition greatly increased the sensitivity of Her2-positive breast cancer cells to the mTOR inhibitor Rapamycin, while it did not increase their sensitivity to Trastuzumab, suggesting that Pin1 might act on Her2 signaling. We found that Pin1 interacted with the protein complex that contains ubiquitinated erbB2 and that Pin1 inhibition accelerated erbB2 degradation, which could be prevented by treatments with the proteasome inhibitor ALLnL.
Conclusion: Pin1 is a novel regulator of erbB2 that modulates the ubiquitin-mediated degradation of erbB2. The overexpression of Pin1 in a majority of Her2-overexpressing breast cancer may contribute to maintain erbB2 levels. Pin1 inhibition alone and in conjunction with mTOR inhibition suppresses the growth of Her2+ breast cancer cells.
Figures



References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Pegram MD, Pauletti G, Slamon DJ. HER-2/neu as a predictive marker of response to breast cancer therapy. Breast Cancer Res Treat. 1998;52:65–77. - PubMed
-
- Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. - PubMed
-
- Wang K, Ma Q, Ren Y, He J, Zhang Y, Chen W. Geldanamycin destabilizes HER2 tyrosine kinase and suppresses Wnt/beta-catenin signaling in HER2 overexpressing human breast cancer cells. Oncol Rep. 2007;17:89–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

