Neural tube derived Wnt signals cooperate with FGF signaling in the formation and differentiation of the trigeminal placodes
- PMID: 19077309
- PMCID: PMC2649920
- DOI: 10.1186/1749-8104-3-35
Neural tube derived Wnt signals cooperate with FGF signaling in the formation and differentiation of the trigeminal placodes
Abstract
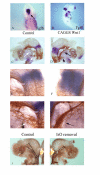
Background: Neurogenic placodes are focal thickenings of the embryonic ectoderm that form in the vertebrate head. It is within these structures that the precursors of the majority of the sensory neurons of the cranial ganglia are specified. The trigeminal placodes, the ophthalmic and maxillomandibular, form close to the midbrain-hindbrain boundary and many lines of evidence have shown that signals emanating from this level of the neuraxis are important for the development of the ophthalmic placode.
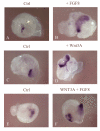
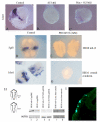
Results: Here, we provide the first evidence that both the ophthalmic and maxillomandibular placodes form under the influence of isthmic Wnt and FGF signals. Activated Wnt signals direct development of the Pax3 expressing ophthalmic placodal field and induce premature differentiation of both the ophthalmic and the maxillomandibular placodes. Similarly, overexpression of Fgf8 directs premature differentiation of the trigeminal placodes. Wnt signals require FGF receptor activity to initiate Pax3 expression and, subsequently, the expression of neural markers, such as Brn3a, within the cranial ectoderm. Furthermore, fibroblast growth factor signaling via the mitogen activated protein kinase pathway is required to maintain early neuronal differentiation within the trigeminal placodes.
Conclusion: We demonstrate the identity of inductive signals that are necessary for trigeminal ganglion formation. This is the first report that describes how isthmic derived Wnt signals act in concert with fibroblast growth factor signaling. Together, both are necessary and sufficient for the establishment and differentiation of the ophthalmic and maxillomandibular placodes and, consequently, the trigeminal ganglion.
Figures





References
-
- Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural tube-ectoderm interactions are required for trigeminal placode formation. Development. 1997;124:4287–4295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

