The role of the RING-finger protein Elfless in Drosophila spermatogenesis and apoptosis
- PMID: 19077536
- PMCID: PMC2668719
- DOI: 10.4161/fly.7352
The role of the RING-finger protein Elfless in Drosophila spermatogenesis and apoptosis
Abstract
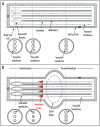
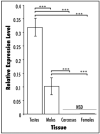
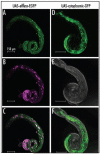
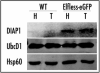
elfless (CG15150, FBgn0032660) maps to polytene region 36DE 5' (left) of reduced ocelli/Pray for Elves (PFE) on chromosome 2L and is predicted to encode a 187 amino acid RING finger E3 ubiquitin ligase that is putatively involved in programmed cell death (PCD, e.g., apoptosis). Several experimental approaches were used to characterize CG15150/elfless and test whether defects in this gene underlie the male sterile phenotype associated with overlapping chromosomal deficiencies of region 36DE. elfless expression is greatly enhanced in the testes and the expression pattern of UAS-elfless-EGFP driven by elfless-Gal4 is restricted to the tail cyst cell nuclei of the testes. Despite this, elfless transgenes failed to rescue the male sterile phenotype in Df/Df flies. Furthermore, null alleles of elfless, generated either by imprecise excision of an upstream P-element or by FLP-FRT deletion between two flanking piggyBac elements, are fertile. In a gain-of-function setting in the eye, we found that elfless genetically interacts with key members of the apoptotic pathway including the initiator caspase Dronc and the ubiquitin conjugating enzyme UbcD1. DIAP1, but not UbcD1, protein levels are increased in heads of flies expressing Elfless-EGFP in the eye, and in testes of flies expressing elfless-Gal4 driven Elfless-EGFP. Based on these findings, we speculate that Elfless may regulate tail cyst cell degradation to provide an advantageous, though not essential, function in the testis.
Figures




References
-
- Castrillon DH, Gönczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135:489–505. - PMC - PubMed
-
- Gönczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114:89–98. - PubMed
-
- Gönczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–47. - PubMed
-
- Fuller MT. Spermatogenesis. In: Bate M, Martinez Arias A, editors. The Development of Drosophila. Cold Spring Harbor Press; Cold Spring Harbor: 1993. pp. 71–147.
-
- Williamson A, Lehmann R. Germ cell development in Drosophila. Annu Rev Cell Dev Biol. 1996;12:365–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
